This is a preprint.
Neuroinvasion of SARS-CoV-2 in human and mouse brain
- PMID: 32935108
- PMCID: PMC7491522
- DOI: 10.1101/2020.06.25.169946
Neuroinvasion of SARS-CoV-2 in human and mouse brain
Update in
-
Neuroinvasion of SARS-CoV-2 in human and mouse brain.J Exp Med. 2021 Mar 1;218(3):e20202135. doi: 10.1084/jem.20202135. J Exp Med. 2021. PMID: 33433624 Free PMC article.
Abstract
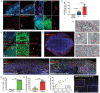
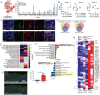
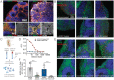
Although COVID-19 is considered to be primarily a respiratory disease, SARS-CoV-2 affects multiple organ systems including the central nervous system (CNS). Yet, there is no consensus whether the virus can infect the brain, or what the consequences of CNS infection are. Here, we used three independent approaches to probe the capacity of SARS-CoV-2 to infect the brain. First, using human brain organoids, we observed clear evidence of infection with accompanying metabolic changes in the infected and neighboring neurons. However, no evidence for the type I interferon responses was detected. We demonstrate that neuronal infection can be prevented either by blocking ACE2 with antibodies or by administering cerebrospinal fluid from a COVID-19 patient. Second, using mice overexpressing human ACE2, we demonstrate in vivo that SARS-CoV-2 neuroinvasion, but not respiratory infection, is associated with mortality. Finally, in brain autopsy from patients who died of COVID-19, we detect SARS-CoV-2 in the cortical neurons, and note pathologic features associated with infection with minimal immune cell infiltrates. These results provide evidence for the neuroinvasive capacity of SARS-CoV2, and an unexpected consequence of direct infection of neurons by SARS-CoV-2.
Conflict of interest statement
Declaration of Interests
None of the authors declare interests related to the manuscript.
Figures


References
-
- Amin N.D., and Pasca S.P. (2018). Building Models of Brain Disorders with Three-Dimensional Organoids. Neuron 100, 389–405. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
