This is a preprint.
Kynurenic acid underlies sex-specific immune responses to COVID-19
- PMID: 32935119
- PMCID: PMC7491534
- DOI: 10.1101/2020.09.06.20189159
Kynurenic acid underlies sex-specific immune responses to COVID-19
Update in
-
Kynurenic acid may underlie sex-specific immune responses to COVID-19.Sci Signal. 2021 Jul 6;14(690):eabf8483. doi: 10.1126/scisignal.abf8483. Sci Signal. 2021. PMID: 34230210 Free PMC article.
Abstract
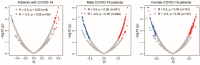
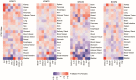
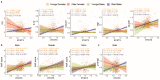
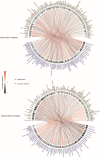
Coronavirus disease-2019 (COVID-19) has poorer clinical outcomes in males compared to females, and immune responses underlie these sex-related differences in disease trajectory. As immune responses are in part regulated by metabolites, we examined whether the serum metabolome has sex-specificity for immune responses in COVID-19. In males with COVID- 19, kynurenic acid (KA) and a high KA to kynurenine (K) ratio was positively correlated with age, inflammatory cytokines, and chemokines and was negatively correlated with T cell responses, revealing that KA production is linked to immune responses in males. Males that clinically deteriorated had a higher KA:K ratio than those that stabilized. In females with COVID-19, this ratio positively correlated with T cell responses and did not correlate with age or clinical severity. KA is known to inhibit glutamate release, and we observed that serum glutamate is lower in patients that deteriorate from COVID-19 compared to those that stabilize, and correlates with immune responses. Analysis of Genotype-Tissue Expression (GTEx) data revealed that expression of kynurenine aminotransferase, which regulates KA production, correlates most strongly with cytokine levels and aryl hydrocarbon receptor activation in older males. This study reveals that KA has a sex-specific link to immune responses and clinical outcomes, in COVID-19 infection.
Conflict of interest statement
Competing interest declaration
The authors declare no competing financial or non-financial interests.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
