Meninges: A Widespread Niche of Neural Progenitors for the Brain
- PMID: 32935634
- PMCID: PMC8442137
- DOI: 10.1177/1073858420954826
Meninges: A Widespread Niche of Neural Progenitors for the Brain
Abstract
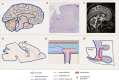
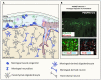
Emerging evidence highlights the several roles that meninges play in relevant brain functions as they are a protective membrane for the brain, produce and release several trophic factors important for neural cell migration and survival, control cerebrospinal fluid dynamics, and embrace numerous immune interactions affecting neural parenchymal functions. Furthermore, different groups have identified subsets of neural progenitors residing in the meninges during development and in the adulthood in different mammalian species, including humans. Interestingly, these immature neural cells are able to migrate from the meninges to the neural parenchyma and differentiate into functional cortical neurons or oligodendrocytes. Immature neural cells residing in the meninges promptly react to brain disease. Injury-induced expansion and migration of meningeal neural progenitors have been observed following experimental demyelination, traumatic spinal cord and brain injury, amygdala lesion, stroke, and progressive ataxia. In this review, we summarize data on the function of meninges as stem cell niche and on the presence of immature neural cells in the meninges, and discuss their roles in brain health and disease. Furthermore, we consider the potential exploitation of meningeal neural progenitors for the regenerative medicine to treat neurological disorders.
Keywords: meninges; neural progenitors; neurogenesis; oligodendrocyte precursor cells; regenerative medicine; stem cell niche.
Conflict of interest statement
Figures






References
-
- Aviezer D, Hecht D, Safran M, Eisinger M, David G, Yayon A.1994. Perlecan, basal lamina proteoglycan, promotes basic fibroblast growth factor-receptor binding, mitogenesis, and angiogenesis. Cell 79(6):1005–13. - PubMed

