Prospective Evaluation of Late-Night Salivary Cortisol and Cortisone by EIA and LC-MS/MS in Suspected Cushing Syndrome
- PMID: 32935666
- PMCID: PMC7480956
- DOI: 10.1210/jendso/bvaa107
Prospective Evaluation of Late-Night Salivary Cortisol and Cortisone by EIA and LC-MS/MS in Suspected Cushing Syndrome
Abstract
Context: Late-night salivary cortisol (LNSC) measured by enzyme immunoassay (EIA-F) is a first-line screening test for Cushing syndrome (CS) with a reported sensitivity and specificity of >90%. However, liquid chromatography-tandem mass spectrometry, validated to measure salivary cortisol (LCMS-F) and cortisone (LCMS-E), has been proposed to be superior diagnostically.
Objective setting and main outcome measures: Prospectively evaluate the diagnostic performance of EIA-F, LCMS-F, and LCMS-E in 1453 consecutive late-night saliva samples from 705 patients with suspected CS.
Design: Patients grouped by the presence or absence of at least one elevated salivary steroid result and then subdivided by diagnosis.
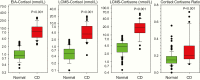
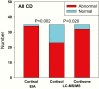
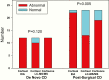
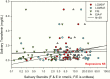
Results: We identified 283 patients with at least one elevated salivary result; 45 had an established diagnosis of neoplastic hypercortisolism (CS) for which EIA-F had a very high sensitivity (97.5%). LCMS-F and LCMS-E had lower sensitivity but higher specificity than EIA-F. EIA-F had poor sensitivity (31.3%) for adrenocorticotropic hormone (ACTH)-independent CS (5 patients with at least 1 and 11 without any elevated salivary result). In patients with Cushing disease (CD), most nonelevated LCMS-F results were in patients with persistent/recurrent CD; their EIA-F levels were lower than in patients with newly diagnosed CD.
Conclusions: Since the majority of patients with ≥1 elevated late-night salivary cortisol or cortisone result did not have CS, a single elevated level has poor specificity and positive predictive value. LNSC measured by EIA is a sensitive test for ACTH-dependent Cushing syndrome but not for ACTH-independent CS. We suggest that neither LCMS-F nor LCMS-E improves the sensitivity of late-night EIA-F for CS.
Keywords: Cushing disease; adrenal Cushing syndrome; assay performance; diagnosis; ectopic ACTH.
© Endocrine Society 2020.
Figures
References
-
- Arnaldi G, Angeli A, Atkinson AB, et al. Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab. 2003;88:5593-5602 - PubMed
-
- Lacroix A, Feelders RA, Stratakis CA, Nieman LK. Cushing’s syndrome. Lancet. 2015;386(9996):913-927. - PubMed
-
- Lonser RR, Nieman L, Oldfield EH. Cushing’s disease: pathobiology, diagnosis, and management. J Neurosurg. 2017;126(2):404-417. - PubMed
-
- Newell-Price J, Bertagna X, Grossman AB, Nieman LK. Cushing’s syndrome. Lancet. 2006;367(9522):1605-1617. - PubMed
LinkOut - more resources
Full Text Sources
