Screening for SARS-CoV-2 antibodies in convalescent plasma in Brazil: Preliminary lessons from a voluntary convalescent donor program
- PMID: 32935877
- PMCID: PMC7756544
- DOI: 10.1111/trf.16065
Screening for SARS-CoV-2 antibodies in convalescent plasma in Brazil: Preliminary lessons from a voluntary convalescent donor program
Abstract
Background: Coronavirus disease 2019 (COVID-19) convalescent plasma (CCP) collection began in two Brazilian hospitals for treatment of severe/critical patients.
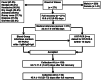
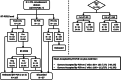
Methods and materials: Mild/moderate COVID-19 convalescents were selected as CCP donors after reverse transcription polymerase chain reaction (RT-PCR) confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and absence of symptoms for ≥14 days plus (a) age (18-60 years), body weight greater than 55 kg; (b) immunohematological studies; (c) no infectious markers of hepatitis B virus, hepatitis C virus, human immunodeficiency virus, human T-lymphotropic virus-1/2, Chagas and syphilis infection; (d) no HLA antibodies (multiparous); (e) second RT-PCR (nasopharyngeal swab and/or blood) negativity; (f) virus neutralization test (cytopathic effect-based virus neutralization test neutralizing antibody) and anti-nucleocapsid protein SARS-CoV-2 IgM, IgG, and IgA enzyme-linked immunosorbent assays.
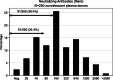
Results: Among 271 donors (41 females, 230 males), 250 presented with neutralizing antibodies. Final RT-PCR was negative on swab (77.0%) or blood (88.4%; P = .46). Final definition of RT-PCR was only defined at more than 28 days after full recovery in 59 of 174 (33.9%) RT-PCR -ve, and 25/69 RT-PCR +ve (36.2%; 13 between 35 and 48 days). Neutralizing antibody titers of 160 or greater were found in 63.6%. Correlation between IgG signal/cutoff of 5.0 or greater and neutralizing antibody of 160 or greater was 82.4%. Combination of final RT-PCR -ve with neutralizing antibody ≥160 was 41.3% (112/271). Serial plasma collection showed decline in neutralizing antibody titers and IgA levels (P < .05), probably denoting a "golden period" for CCP collection (≤28 days after joining the program); IgA might have an important role as neutralizing antibody. Donor's weight, days between disease onset and serial plasma collection, and IgG and IgM levels are important predictors for neutralizing antibody titer.
Conclusions: RT-PCR +ve cases are still detected in 36.2% within 28 to 48 days after recovery. High anti-nucleocapsid protein IgG levels may be used as a surrogate marker to neutralizing antibody.
Keywords: COVID-19; SARS-COV-2; coronavirus; convalescent plasma therapy; passive immune therapy.
© 2020 The Authors. Transfusion published by Wiley Periodicals LLC. on behalf of AABB.
Conflict of interest statement
C.P.S. is funded by Grant 2018/23680‐0 (Fundação de. Amparo à Pesquisa do Estado de São Paulo); D.B.A. by Grant 88 887.131387/2016‐00 (Coordenação de Aperfeiçoamento de Pessoal de. Nível Superior ‐ CAPES), R.R.G.M. by Grant 2017/24769‐2 (Fundação de. Amparo à Pesquisa do Estado de São Paulo) and E.L.D. by Grants 2016/20045‐7 and 2020/06409‐1 (Fundação de Amparo à Pesquisa do Estado de São Paulo). All other authors have no conflict of interest.
Figures




Similar articles
-
Persistence of SARS-CoV-2 nasopharyngeal swab PCR positivity in COVID-19 convalescent plasma donors.Transfusion. 2020 Dec;60(12):2962-2968. doi: 10.1111/trf.16015. Epub 2020 Aug 24. Transfusion. 2020. PMID: 32840002 Free PMC article.
-
The effect of early COVID-19 treatment with convalescent plasma on antibody responses to SARS-CoV-2.Microbiol Spectr. 2025 Jul;13(7):e0300624. doi: 10.1128/spectrum.03006-24. Epub 2025 Jun 9. Microbiol Spectr. 2025. PMID: 40488473 Free PMC article. Clinical Trial.
-
A longitudinal study of convalescent plasma (CCP) donors and correlation of ABO group, initial neutralizing antibodies (nAb), and body mass index (BMI) with nAb and anti-nucleocapsid (NP) SARS-CoV-2 antibody kinetics: Proposals for better quality of CCP collections.Transfusion. 2021 May;61(5):1447-1460. doi: 10.1111/trf.16323. Epub 2021 Feb 19. Transfusion. 2021. PMID: 33604884 Free PMC article.
-
Clinical predictors of SARS-CoV-2 neutralizing antibody titers in COVID-19 convalescents: Implications for convalescent plasma donor recruitment.Eur J Haematol. 2021 Jul;107(1):24-28. doi: 10.1111/ejh.13630. Epub 2021 Apr 20. Eur J Haematol. 2021. PMID: 33780551 Free PMC article. Review.
-
Antibody Responses in COVID-19: A Review.Front Immunol. 2021 Apr 15;12:633184. doi: 10.3389/fimmu.2021.633184. eCollection 2021. Front Immunol. 2021. PMID: 33936045 Free PMC article. Review.
Cited by
-
COVID-19 convalescent plasma cohort study: Evaluation of the association between both donor and recipient neutralizing antibody titers and patient outcomes.Transfusion. 2021 Aug;61(8):2295-2306. doi: 10.1111/trf.16573. Epub 2021 Jul 8. Transfusion. 2021. PMID: 34173248 Free PMC article.
-
International Forum on the Collection and Use of COVID-19 Convalescent Plasma: Responses.Vox Sang. 2021 Nov;116(10):e71-e120. doi: 10.1111/vox.13114. Epub 2021 May 20. Vox Sang. 2021. PMID: 34013981 Free PMC article. No abstract available.
-
Antibody Response After SARS-CoV-2 Infection and Implications for Immunity : A Rapid Living Review.Ann Intern Med. 2021 Jun;174(6):811-821. doi: 10.7326/M20-7547. Epub 2021 Mar 16. Ann Intern Med. 2021. PMID: 33721517 Free PMC article. Review.
-
Immunodominant antibody responses directed to SARS-CoV-2 hotspot mutation sites and risk of immune escape.Front Immunol. 2023 Jan 5;13:1010105. doi: 10.3389/fimmu.2022.1010105. eCollection 2022. Front Immunol. 2023. PMID: 36685521 Free PMC article.
-
Tonsils are major sites of persistence of SARS-CoV-2 in children.Microbiol Spectr. 2023 Sep 22;11(5):e0134723. doi: 10.1128/spectrum.01347-23. Online ahead of print. Microbiol Spectr. 2023. PMID: 37737615 Free PMC article.
References
-
- World Health Organization Naming the coronavirus disease (COVID‐19) and the virus that causes it. Available at https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/technica.... Accessed June 2, 2020.
-
- World Health Organization (WHO) Clinical management of COVID‐19. Interim guidance 27 May, 2020 – WHO/2019‐nCoV/clinical/2020.5. https://www.who.int/publications-detail/clinical-management-of-covid-19. Accessed August 2, 2020.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

