Isolable Silicon-Based Polycations with Lewis Superacidity
- PMID: 32935903
- PMCID: PMC7756528
- DOI: 10.1002/anie.202011696
Isolable Silicon-Based Polycations with Lewis Superacidity
Abstract
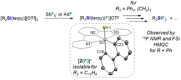
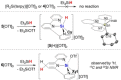
Molecular silicon polycations of the types R2 Si2+ and RSi3+ (R=H, organic groups) are elusive Lewis superacids and currently unknown in the condensed phase. Here, we report the synthesis of a series of isolable terpyridine-stabilized R2 Si2+ and RSi3+ complexes, [R2 Si(terpy)]2+ (R=Ph 12+ ; R2 =C12 H8 22+ , (CH2 )3 32+ ) and [RSi(terpy)]3+ (R=Ph 43+ , cyclohexyl 53+ , m-xylyl 63+ ), in form of their triflate salts. The stabilization of the latter is achieved through higher coordination and to the expense of reduced fluoride-ion affinities, but a significant level of Lewis superacidity is nonetheless retained as verified by theory and experiment. The complexes activate C(sp3 )-F bonds, as showcased by stoichiometric fluoride abstraction from 1-fluoroadamantane (AdF) and the catalytic hydrodefluorination of AdF. The formation of the crystalline adducts [2(F)]+ and [5(H)]2+ documents in particular the high reactivity towards fluoride and hydride donors.
Keywords: Coordination compounds; Hydride transfer; Hydrodefluorination; Main group elements; Silanes.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Silicon Tetrakis(trifluoromethanesulfonate): A Simple Neutral Silane Acting as a Soft and Hard Lewis Superacid.Angew Chem Int Ed Engl. 2021 Jun 7;60(24):13656-13660. doi: 10.1002/anie.202103414. Epub 2021 May 5. Angew Chem Int Ed Engl. 2021. PMID: 33826216 Free PMC article.
-
Bipyridinium and Phenanthrolinium Dications for Metal-Free Hydrodefluorination: Distinctive Carbon-Based Reactivity.Chemistry. 2021 Aug 11;27(45):11730-11737. doi: 10.1002/chem.202101534. Epub 2021 Jul 8. Chemistry. 2021. PMID: 34107119
-
Bis(catecholato)silanes: assessing, rationalizing and increasing silicon's Lewis superacidity.Chem Sci. 2019 Jun 17;10(31):7379-7388. doi: 10.1039/c9sc02167a. eCollection 2019 Aug 21. Chem Sci. 2019. PMID: 31489160 Free PMC article.
-
Lewis Superacids: Classifications, Candidates, and Applications.Chemistry. 2018 Dec 5;24(68):17881-17896. doi: 10.1002/chem.201802698. Epub 2018 Oct 19. Chemistry. 2018. PMID: 29943864 Review.
-
Recent Advances in the Chemistry of Heavier Group 14 Analogues of Carbonyls.Chem Asian J. 2022 Sep 14;17(18):e202200611. doi: 10.1002/asia.202200611. Epub 2022 Aug 12. Chem Asian J. 2022. PMID: 35883252 Review.
Cited by
-
Cationic Carbene Analogues: Donor-Free Phosphenium and Arsenium Ions.Angew Chem Int Ed Engl. 2021 Aug 23;60(35):19133-19138. doi: 10.1002/anie.202107975. Epub 2021 Jul 26. Angew Chem Int Ed Engl. 2021. PMID: 34219354 Free PMC article.
-
Intramolecular donor-stabilized tetra-coordinated germanium(iv) di-cations and their Lewis acidic properties.Chem Sci. 2023 Nov 16;14(47):13755-13764. doi: 10.1039/d3sc03717g. eCollection 2023 Dec 6. Chem Sci. 2023. PMID: 38075658 Free PMC article.
-
Silicon Tetrakis(trifluoromethanesulfonate): A Simple Neutral Silane Acting as a Soft and Hard Lewis Superacid.Angew Chem Int Ed Engl. 2021 Jun 7;60(24):13656-13660. doi: 10.1002/anie.202103414. Epub 2021 May 5. Angew Chem Int Ed Engl. 2021. PMID: 33826216 Free PMC article.
-
Ga+-catalyzed hydrosilylation? About the surprising system Ga+/HSiR3/olefin, proof of oxidation with subvalent Ga+ and silylium catalysis with perfluoroalkoxyaluminate anions.Chem Sci. 2021 Nov 23;13(2):439-453. doi: 10.1039/d1sc05331k. eCollection 2022 Jan 5. Chem Sci. 2021. PMID: 35126976 Free PMC article.
References
-
- None
-
- Sereda O., Tabassum S., Wilhelm R. in Topics in Current Chemistry (Ed.: List B.), Springer, Heidelberg, 2009, pp. 349–393; - PubMed
-
- Weicker S. A., Stephan D. W., Bull. Chem. Soc. Jpn. 2015, 88, 1003–1016;
-
- Davydova E. I., Sevastianova T. N., Timoshkin A. Y., Coord. Chem. Rev. 2015, 297–298, 91–126;
-
- Stephan D. W., Erker G., Angew. Chem. Int. Ed. 2010, 49, 46–76; - PubMed
- Angew. Chem. 2010, 122, 50–81.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

