Co-drug delivery of regorafenib and cisplatin with amphiphilic copolymer nanoparticles: enhanced in vivo antitumor cancer therapy in nursing care
- PMID: 32936009
- PMCID: PMC7534345
- DOI: 10.1080/10717544.2020.1815897
Co-drug delivery of regorafenib and cisplatin with amphiphilic copolymer nanoparticles: enhanced in vivo antitumor cancer therapy in nursing care
Abstract
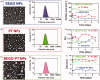
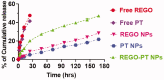
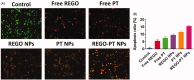
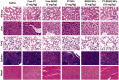
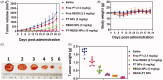
Cancers continue to be the second leading cause of death worldwide. Despite the development and improvement of surgery, chemotherapy and radiotherapy in cancer management, effective tumor ablation strategies are still in need due to high cancer patient mortality. Hence, we have established a new approach to achieve treatment-actuated modifications in a tumor microenvironment by using synergistic activity between two potential anticancer drugs. Dual drug delivery of Regorafenib (REGO) and Cisplatin (PT) exhibits a great anticancer potential, as REGO enhances the effect of PT treatment of human cells by providing stability of the microenvironment. However, encapsulation of REGO and PT fanatical by methoxypoly(ethylene glycol)-block-poly(D, L-lactic acid) (PEG-PLA in termed as NPs) is incompetent owing to unsuitability between the binary Free REGO and PT core and the polymeric system. Now, we display that PT can be prepared by hydrophobic coating of the dual drug centers with dioleoylphosphatidic acid (DOPA). The DOPA-covered PT can be co-encapsulated in PLGA NPs alongside REGO to stimulate excellent anticancer property. The occurrence of the PT suggestively enhanced the encapsulations of REGO into PLGA NPs (REGO-PT NPs). Further, the morphology of REGO NPs, PT NPs, and REGO-PT NPs and nanoparticle size was examined by transmission microscopy (TEM), respectively. Furthermore REGO-PT NPs induced significant apoptosis in human lung A549 and ovarian A2780 cancer cells by in vitro. The morphological observation and apoptosis were confirmed by the various biochemical assayes (AO-EB, Nuclear Staining and Annexin V-FITC). In a xenograft model of lung cancer, this nanotherapy shows a durable inhibition of tumor progression upon the administration of a tolerable dose. Our results suggest that a hydrophobic and highly toxic drug can be rationally converted into a pharmacologically efficient and self-deliverable nursing care of nanotherapy. Highlights Dual drug delivery of Regorafenib (REGO) and Cisplatin (PT) exhibits a great anticancer potential, as REGO enhances the effect of PT treatment of human cells by providing stability of the microenvironment. REGO-PT NPs induced significant apoptosis in human lung A549 and ovarian A2780 cancer cells by in vitro. The morphological observation and apoptosis were confirmed by the various biochemical assayes. In a xenograft model of lung cancer, this nanotherapy shows a durable inhibition of tumor progression upon the administration of a tolerable dose.
Keywords: Combinational delivery; apoptosis; cancer; in vivo antitumor efficacy.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

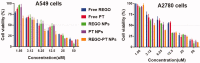


Similar articles
-
Precise engineering of hybrid molecules-loaded macromolecular nanoparticles shows in vitro and in vivo antitumor efficacy toward the treatment of nasopharyngeal cancer cells.Drug Deliv. 2021 Dec;28(1):776-786. doi: 10.1080/10717544.2021.1902022. Drug Deliv. 2021. PMID: 33866910 Free PMC article.
-
Combinational dual drug delivery system to enhance the care and treatment of gastric cancer patients.Drug Deliv. 2020 Dec;27(1):1491-1500. doi: 10.1080/10717544.2020.1822460. Drug Deliv. 2020. PMID: 33100060 Free PMC article.
-
Co-delivery of cisplatin and paclitaxel by folic acid conjugated amphiphilic PEG-PLGA copolymer nanoparticles for the treatment of non-small lung cancer.Oncotarget. 2015 Dec 8;6(39):42150-68. doi: 10.18632/oncotarget.6243. Oncotarget. 2015. PMID: 26517524 Free PMC article.
-
Nanoparticle-Mediated Combination Therapy: Two-in-One Approach for Cancer.Int J Mol Sci. 2018 Oct 20;19(10):3264. doi: 10.3390/ijms19103264. Int J Mol Sci. 2018. PMID: 30347840 Free PMC article. Review.
-
Innovative nanoparticle strategies for treating oral cancers.Med Oncol. 2025 Apr 26;42(6):182. doi: 10.1007/s12032-025-02728-y. Med Oncol. 2025. PMID: 40285805 Review.
Cited by
-
Chemo-radiotherapy with 177Lu-PLGA(RGF)-CXCR4L for the targeted treatment of colorectal cancer.Front Med (Lausanne). 2023 Jun 12;10:1191315. doi: 10.3389/fmed.2023.1191315. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37378300 Free PMC article.
-
Combination of Quercetin or/and siRNA-loaded DDAB-mPEG-PCL hybrid nanoparticles reverse resistance to Regorafenib in colon cancer cells.BMC Complement Med Ther. 2022 Dec 27;22(1):340. doi: 10.1186/s12906-022-03787-8. BMC Complement Med Ther. 2022. PMID: 36575448 Free PMC article.
-
Co-delivery systems: hope for clinical application?Drug Deliv Transl Res. 2022 Jun;12(6):1339-1354. doi: 10.1007/s13346-021-01041-1. Epub 2021 Aug 16. Drug Deliv Transl Res. 2022. PMID: 34402023 Review.
-
Pulmonary route of administration is instrumental in developing therapeutic interventions against respiratory diseases.Saudi Pharm J. 2020 Dec;28(12):1655-1665. doi: 10.1016/j.jsps.2020.10.012. Epub 2020 Nov 4. Saudi Pharm J. 2020. Retraction in: Saudi Pharm J. 2022 May;30(5):646. doi: 10.1016/j.jsps.2022.04.011. PMID: 33424258 Free PMC article. Retracted. Review.
References
-
- AARON WOLD (1971). Platinum metal chalcogenides, In: Platinum group metals and compounds, U. V. Rao (Ed.). Washington, D.C: American Chemical Society, p. 2–17.
-
- Ambrogio MW, Frasconi M, Yilmaz MD, Chen X. (2013). New methods for improved characterization of silica nanoparticle-based drug delivery systems. Langmuir 29:15386–93. - PubMed
-
- Balaji S, Mohamed Subarkhan MK, Ramesh R, et al. (2020). Synthesis and structure of arene Ru(II) N∧O-chelating complexes: in vitro cytotoxicity and cancer cell death mechanism. Organometallics 39:1366–75.
-
- Boyd P, Variano B, Spence P, et al. (2019). In vitro release testing methods for drug-releasing vaginal rings. J Control Release 313:54–69. - PubMed
-
- Cao Z-T, Chen Z-Y, Sun C-Y, et al. (2016). Overcoming tumor resistance to cisplatin by cationic lipid-assisted prodrug nanoparticles. Biomaterials 94:9–19. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
