The Role of Chondroitin Sulfate Proteoglycans in Nervous System Development
- PMID: 32936033
- PMCID: PMC7780190
- DOI: 10.1369/0022155420959147
The Role of Chondroitin Sulfate Proteoglycans in Nervous System Development
Abstract
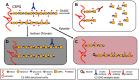
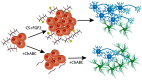
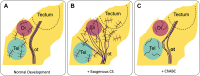
The orderly development of the nervous system is characterized by phases of cell proliferation and differentiation, neural migration, axonal outgrowth and synapse formation, and stabilization. Each of these processes is a result of the modulation of genetic programs by extracellular cues. In particular, chondroitin sulfate proteoglycans (CSPGs) have been found to be involved in almost every aspect of this well-orchestrated yet delicate process. The evidence of their involvement is complex, often contradictory, and lacking in mechanistic clarity; however, it remains obvious that CSPGs are key cogs in building a functional brain. This review focuses on current knowledge of the role of CSPGs in each of the major stages of neural development with emphasis on areas requiring further investigation.
Keywords: axon guidance; extracellular matrix; glycosaminoglycans; neural migration; neurogenesis; proteoglycans; synapse formation.
Conflict of interest statement
Figures


References
-
- Yamaguchi Y. Heparan sulfate proteoglycans in the nervous system: their diverse roles in neurogenesis, axon guidance, and synaptogenesis. Semin Cell Dev Biol. 2001;12(2):99–106. - PubMed
-
- Melrose J. Keratan sulfate (KS)-proteoglycans and neuronal regulation in health and disease: the importance of KS-glycodynamics and interactive capability with neuroregulatory ligands. J Neurochem. 2019;149(2):170–94. - PubMed
-
- Kitagawa H, Uyama T, Sugahara K. Molecular cloning and expression of a human chondroitin synthase. J Biol Chem. 2001;276(42):38721–6. - PubMed
-
- Yada T, Gotoh M, Sato T, Shionyu M, Go M, Kaseyama H, Iwasaki H, Kikuchi N, Kwon YD, Togayachi A, Kudo T, Watanabe H, Narimatsu H, Kimata K. Chondroitin sulfate synthase-2. Molecular cloning and characterization of a novel human glycosyltransferase homologous to chondroitin sulfate glucuronyltransferase, which has dual enzymatic activities. J Biol Chem. 2003;278(32):30235–47. - PubMed
-
- Yada T, Sato T, Kaseyama H, Gotoh M, Iwasaki H, Kikuchi N, Kwon YD, Togayachi A, Kudo T, Watanabe H, Narimatsu H, Kimata K. Chondroitin sulfate synthase-3. Molecular cloning and characterization. J Biol Chem. 2003;278(41):39711–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

