Optogenetic activation of heterotrimeric G-proteins by LOV2GIVe, a rationally engineered modular protein
- PMID: 32936073
- PMCID: PMC7515630
- DOI: 10.7554/eLife.60155
Optogenetic activation of heterotrimeric G-proteins by LOV2GIVe, a rationally engineered modular protein
Abstract
Heterotrimeric G-proteins are signal transducers involved in mediating the action of many natural extracellular stimuli and many therapeutic agents. Non-invasive approaches to manipulate the activity of G-proteins with high precision are crucial to understand their regulation in space and time. Here, we developed LOV2GIVe, an engineered modular protein that allows the activation of heterotrimeric G-proteins with blue light. This optogenetic construct relies on a versatile design that differs from tools previously developed for similar purposes, that is metazoan opsins, which are light-activated G-protein-coupled receptors (GPCRs). Instead, LOV2GIVe consists of the fusion of a G-protein activating peptide derived from a non-GPCR regulator of G-proteins to a small plant protein domain, such that light uncages the G-protein activating module. Targeting LOV2GIVe to cell membranes allowed for light-dependent activation of Gi proteins in different experimental systems. In summary, LOV2GIVe expands the armamentarium and versatility of tools available to manipulate heterotrimeric G-protein activity.
Keywords: E. coli; GEF; GIV; GPCR; GTPase; Girdin; Optogenetics; S. cerevisiae; biochemistry; cell biology; chemical biology; human.
© 2020, Garcia-Marcos et al.
Conflict of interest statement
MG, KP, AM, MM, AL, LN No competing interests declared
Figures





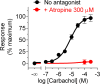
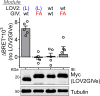



Similar articles
-
Heterotrimeric G protein signaling without GPCRs: The Gα-binding-and-activating (GBA) motif.J Biol Chem. 2024 Mar;300(3):105756. doi: 10.1016/j.jbc.2024.105756. Epub 2024 Feb 15. J Biol Chem. 2024. PMID: 38364891 Free PMC article. Review.
-
Specific inhibition of GPCR-independent G protein signaling by a rationally engineered protein.Proc Natl Acad Sci U S A. 2017 Nov 28;114(48):E10319-E10328. doi: 10.1073/pnas.1707992114. Epub 2017 Nov 13. Proc Natl Acad Sci U S A. 2017. PMID: 29133411 Free PMC article.
-
Membrane Recruitment of the Non-receptor Protein GIV/Girdin (Gα-interacting, Vesicle-associated Protein/Girdin) Is Sufficient for Activating Heterotrimeric G Protein Signaling.J Biol Chem. 2016 Dec 30;291(53):27098-27111. doi: 10.1074/jbc.M116.764431. Epub 2016 Nov 18. J Biol Chem. 2016. PMID: 27864364 Free PMC article.
-
Engineering a V(2) vasopressin receptor agonist- and regulator of G-protein-signaling-sensitive G protein.Anal Biochem. 2002 Jan 15;300(2):212-20. doi: 10.1006/abio.2001.5448. Anal Biochem. 2002. PMID: 11779113
-
The GAPs, GEFs, GDIs and…now, GEMs: New kids on the heterotrimeric G protein signaling block.Cell Cycle. 2017 Apr 3;16(7):607-612. doi: 10.1080/15384101.2017.1282584. Epub 2017 Mar 13. Cell Cycle. 2017. PMID: 28287365 Free PMC article. Review.
Cited by
-
Complementary biosensors reveal different G-protein signaling modes triggered by GPCRs and non-receptor activators.Elife. 2021 Mar 31;10:e65620. doi: 10.7554/eLife.65620. Elife. 2021. PMID: 33787494 Free PMC article.
-
Building unconventional G protein-coupled receptors, one block at a time.Trends Pharmacol Sci. 2021 Jul;42(7):514-517. doi: 10.1016/j.tips.2021.04.005. Epub 2021 May 10. Trends Pharmacol Sci. 2021. PMID: 33985816 Free PMC article.
-
Fine-tuning GPCR-mediated neuromodulation by biasing signaling through different G protein subunits.Mol Cell. 2023 Jul 20;83(14):2540-2558.e12. doi: 10.1016/j.molcel.2023.06.006. Epub 2023 Jun 29. Mol Cell. 2023. PMID: 37390816 Free PMC article.
-
Heterotrimeric G protein signaling without GPCRs: The Gα-binding-and-activating (GBA) motif.J Biol Chem. 2024 Mar;300(3):105756. doi: 10.1016/j.jbc.2024.105756. Epub 2024 Feb 15. J Biol Chem. 2024. PMID: 38364891 Free PMC article. Review.
-
AGS3-based optogenetic GDI induces GPCR-independent Gβγ signaling and macrophage migration.bioRxiv [Preprint]. 2024 Jun 5:2024.06.04.597473. doi: 10.1101/2024.06.04.597473. bioRxiv. 2024. Update in: Open Biol. 2025 Feb;15(2):240181. doi: 10.1098/rsob.240181. PMID: 38895415 Free PMC article. Updated. Preprint.
References
-
- Aznar N, Midde KK, Dunkel Y, Lopez-Sanchez I, Pavlova Y, Marivin A, Barbazán J, Murray F, Nitsche U, Janssen KP, Willert K, Goel A, Abal M, Garcia-Marcos M, Ghosh P. Daple is a novel non-receptor GEF required for trimeric G protein activation in wnt signaling. eLife. 2015;4:e07091. doi: 10.7554/eLife.07091. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

