GTF2IRD1 overexpression promotes tumor progression and correlates with less CD8+ T cells infiltration in pancreatic cancer
- PMID: 32936232
- PMCID: PMC7527428
- DOI: 10.1042/BSR20202150
GTF2IRD1 overexpression promotes tumor progression and correlates with less CD8+ T cells infiltration in pancreatic cancer
Abstract
Background: General Transcription Factor II-I Repeat Domain-Containing Protein 1 (GTF2IRD1) is a member of the GTF21 gene family, which encodes a set of multifunctional transcription factors. However, the potential function of GTF2IRD1 in pancreatic cancer (PC) still remains unknown. Study on GTF2IRD1 might provide a new insight into the carcinogenesis and therapeutics of PC.
Methods: In the current study, the clinical significance and potential biological of GTF2IRD1 were evaluated by bioinformatics analysis. The oncogenic role of GTF2IRD1 in PC was also determined using in vitro studies. Possible associations between GTF2IRD1 expression and tumor immunity were analyzed using ESTIMATE algorithm and single-sample Gene Set Enrichment Analysis (ssGSEA).
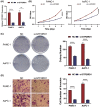
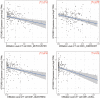
Results: GTF2IRD1 expression was significantly up-regulated in tumor tissues, and positively associated with higher histologic grade, higher American Joint Committee on Cancer (AJCC) stage, and worse prognosis. Function enrichment analysis demonstrated that GTF2IRD1 may be involved in pancreatic adenocarcinoma pathway, TGF-β signaling pathway, and tumor-infiltrating lymphocyte (TIL) related biological functions, such as T-cell receptor signaling pathway, leukocyte transendothelial migration, resistin as a regulator of inflammation, and regulation of leukocyte-mediated cytotoxicity. Knockdown of GTF2IRD1 expression inhibited cancer cell proliferation, colony formation, and invasion in vitro. ESTIMATE algorithm and ssGSEA demonstrated that GTF2IRD1 expression negatively correlated with the infiltration and anti-tumor activity of TILs, especially for CD8+ T cells.
Conclusion: The study demonstrates that GTF2IRD1 overexpression promotes tumor progression and correlates with less CD8+ T cells infiltration in PC.
Keywords: CD8+ T cells; GTF2IRD1; T cell receptor signaling pathway; TGF-β; pancreatic cancer.
© 2020 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures


References
-
- Schouwenburg M.G., Suijkerbuijk K.P.M., Koornstra R.H.T., Jochems A., van Zeijl M.C.T., van den Eertwegh A.J.M. et al. (2019) Switching to immune checkpoint inhibitors upon response to targeted therapy; the road to long-term survival in advanced melanoma patients with highly elevated serum LDH? Cancers (Basel) 11, 1940 10.3390/cancers11121940 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

