Association of UGT1A1*6 polymorphism with irinotecan-based chemotherapy reaction in colorectal cancer patients: a systematic review and a meta-analysis
- PMID: 32936306
- PMCID: PMC7578622
- DOI: 10.1042/BSR20200576
Association of UGT1A1*6 polymorphism with irinotecan-based chemotherapy reaction in colorectal cancer patients: a systematic review and a meta-analysis
Abstract
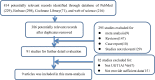
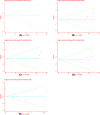
Colorectal cancer (CRC) is a leading cause of cancer-related deaths across the world. Irinotecan (IRI) is commonly used to treat CRC, and IRI-based chemotherapy is linked with adverse reaction and the efficacy of the treatment regimen. The gene UGT1A1 plays a central role in the IRI metabolic pathway. A polymorphism UGT1A1*6 has been widely researched which may be related to response of IRI-based chemotherapy in CRC. All relevant studies were strictly searched from PubMed, Embase, Cochrane Library and Web of Science databases to explore the associations between UGT1A1*6 and response of IRI-based chemotherapy with CRC. Nine articles comprising 1652 patients were included in the final combination. Meta-analysis showed G allele or GG had a lower risk of severe late-onset diarrhea compared with A/AA in allele model and homozygote model (G vs. A: OR = 0.53, 95% CI: 0.28-0.99, P=0.05; GG vs. AA: OR = 0.48, 95% CI: 0.23-0.99, P=0.05), no significant association was observed in other models. In addition, a significant association between UGT1A1*6 and neutropenia was observed in all models (G vs. A: OR = 0.57, 95% CI: 0.46-0.71, P=0.00; GG vs. AA: OR = 0.28, 95% CI: 0.17-0.45, P=0.01; GA vs. AA: OR = 0.42, 95% CI: 0.26-0.70, P=0.00; GG+GA vs. AA: OR = 0.32, 95% CI: 0.20-0.52, P=0.00; GG vs. AA+GA: OR = 0.40, 95% CI: 0.22-0.71, P=0.00), whereas, no relationship was found between UGT1A1*6 and clinical response among the different genotypes. UGT1A1*6 may be considered as a biomarker for IRI-based chemotherapy in CRC.
Keywords: UGT1A1; colorectal cancer; irinotecan; meta-analysis; response; rs4148323.
© 2020 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures









References
-
- Cremolini C., Rossini D., Dell’Aquila E., Lonardi S., Conca E., Del Re M. et al. (2019) Rechallenge for patients with RAS and BRAF wild-type metastatic colorectal cancer with acquired resistance to first-line cetuximab and irinotecan: a phase 2 single-arm clinical trial. JAMA Oncol. 5, 343–350 10.1001/jamaoncol.2018.5080 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

