Targeted IgMs agonize ocular targets with extended vitreal exposure
- PMID: 32936727
- PMCID: PMC7577241
- DOI: 10.1080/19420862.2020.1818436
Targeted IgMs agonize ocular targets with extended vitreal exposure
Abstract
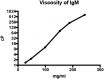
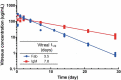
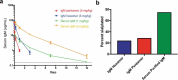
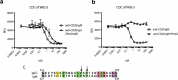
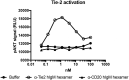
Treatment of ocular disease is hindered by the presence of the blood-retinal barrier, which restricts access of systemic drugs to the eye. Intravitreal injections bypass this barrier, delivering high concentrations of drug to the targeted tissue. However, the recommended dosing interval for approved biologics is typically 6-12 weeks, and frequent travel to the physician's office poses a substantial burden for elderly patients with poor vision. Real-world data suggest that many patients are under-treated. Here, we investigate IgMs as a novel platform for treating ocular disease. We show that IgMs are well-suited to ocular administration due to moderate viscosity, long ocular exposure, and rapid systemic clearance. The complement-dependent cytotoxicity of IgMs can be readily removed with a P436G mutation, reducing safety liabilities. Furthermore, dodecavalent binding of IgM hexamers can potently activate pathways implicated in the treatment of progressive blindness, including the Tie2 receptor tyrosine kinase signaling pathway for the treatment of diabetic macular edema, or the death receptor 4 tumor necrosis family receptor pathway for the treatment of wet age-related macular degeneration. Collectively, these data demonstrate the promise of IgMs as therapeutic agonists for treating progressive blindness.
Keywords: IgM; agonism; antibody engineering; long-acting delivery; ocular therapeutics.
Figures

Similar articles
-
Drug delivery strategies for combination ophthalmic treatments.Retina. 2009 Jun;29(6 Suppl):S24-6. doi: 10.1097/IAE.0b013e3181ad2463. Retina. 2009. PMID: 19553793
-
Three-Dimensional Transport Model for Intravitreal and Suprachoroidal Drug Injection.Invest Ophthalmol Vis Sci. 2018 Oct 1;59(12):5266-5276. doi: 10.1167/iovs.17-23632. Invest Ophthalmol Vis Sci. 2018. PMID: 30383198 Free PMC article.
-
Novel drug delivery systems for retinal diseases. A review.Ophthalmic Res. 2009;41(3):124-35. doi: 10.1159/000209665. Epub 2009 Mar 26. Ophthalmic Res. 2009. PMID: 19321933 Review.
-
A new era in posterior segment ocular drug delivery: Translation of systemic, cell-targeted, dendrimer-based therapies.Adv Drug Deliv Rev. 2023 Sep;200:115005. doi: 10.1016/j.addr.2023.115005. Epub 2023 Jul 6. Adv Drug Deliv Rev. 2023. PMID: 37419213 Review.
-
Clinical evidence of intravitreal triamcinolone acetonide in the management of age-related macular degeneration.Curr Drug Targets. 2011 Feb;12(2):149-72. doi: 10.2174/138945011794182746. Curr Drug Targets. 2011. PMID: 20887246 Review.
Cited by
-
Agonist antibody discovery: Experimental, computational, and rational engineering approaches.Drug Discov Today. 2022 Jan;27(1):31-48. doi: 10.1016/j.drudis.2021.09.008. Epub 2021 Sep 24. Drug Discov Today. 2022. PMID: 34571277 Free PMC article. Review.
-
Multimeric Anti-DR5 IgM Agonist Antibody IGM-8444 Is a Potent Inducer of Cancer Cell Apoptosis and Synergizes with Chemotherapy and BCL-2 Inhibitor ABT-199.Mol Cancer Ther. 2021 Dec;20(12):2483-2494. doi: 10.1158/1535-7163.MCT-20-1132. Epub 2021 Oct 28. Mol Cancer Ther. 2021. PMID: 34711645 Free PMC article.
-
Avidity in antibody effector functions and biotherapeutic drug design.Nat Rev Drug Discov. 2022 Oct;21(10):715-735. doi: 10.1038/s41573-022-00501-8. Epub 2022 Jul 5. Nat Rev Drug Discov. 2022. PMID: 35790857 Free PMC article. Review.
References
-
- Congdon N, O’Colmain B, Klaver CCW, Klein R, Muñoz B, Friedman DS, Kempen J, Taylor HR, Mitchell P, Eye Diseases Prevalence Research Group . Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–13. - PubMed
-
- Brown DM, Nguyen QD, Marcus DM, Boyer DS, Patel S, Feiner L, Schlottmann PG, Rundle AC, Zhang J, Rubio RG, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials. Ophthalmology. 2013;120:2013–22. doi: 10.1016/j.ophtha.2013.02.034. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
