Rapid hematoma growth triggers spreading depolarizations in experimental intracortical hemorrhage
- PMID: 32936730
- PMCID: PMC8142136
- DOI: 10.1177/0271678X20951993
Rapid hematoma growth triggers spreading depolarizations in experimental intracortical hemorrhage
Abstract
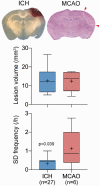
Recurrent waves of spreading depolarization (SD) occur in brain injury and are thought to affect outcomes. What triggers SD in intracerebral hemorrhage is poorly understood. We employed intrinsic optical signaling, laser speckle flowmetry, and electrocorticography to elucidate the mechanisms triggering SD in a collagenase model of intracortical hemorrhage in mice. Hematoma growth, SD occurrence, and cortical blood flow changes were tracked. During early hemorrhage (0-4 h), 17 out of 38 mice developed SDs, which always originated from the hematoma. No SD was detected at late time points (8-52 h). Neither hematoma size, nor peri-hematoma perfusion were associated with SD occurrence. Further, arguing against ischemia as a trigger factor, normobaric hyperoxia did not inhibit SD occurrence. Instead, SDs always occurred during periods of rapid hematoma growth, which was two-fold faster immediately preceding an SD compared with the peak growth rates in animals that did not develop any SDs. Induced hypertension accelerated hematoma growth and resulted in a four-fold increase in SD occurrence compared with normotensive animals. Altogether, our data suggest that spontaneous SDs in this intracortical hemorrhage model are triggered by the mechanical distortion of tissue by rapidly growing hematomas.
Keywords: Cerebral amyloid angiopathy; electrocorticography; intracerebral hemorrhage; laser speckle imaging; spreading depolarization.
Conflict of interest statement
Figures






Similar articles
-
Spreading Depolarizations Suppress Hematoma Growth in Hyperacute Intracerebral Hemorrhage in Mice.Stroke. 2023 Oct;54(10):2640-2651. doi: 10.1161/STROKEAHA.123.042632. Epub 2023 Aug 23. Stroke. 2023. PMID: 37610105 Free PMC article.
-
Requisite ischemia for spreading depolarization occurrence after subarachnoid hemorrhage in rodents.J Cereb Blood Flow Metab. 2017 May;37(5):1829-1840. doi: 10.1177/0271678X16659303. Epub 2016 Jan 1. J Cereb Blood Flow Metab. 2017. PMID: 27432225 Free PMC article.
-
Simulation of spreading depolarization trajectories in cerebral cortex: Correlation of velocity and susceptibility in patients with aneurysmal subarachnoid hemorrhage.Neuroimage Clin. 2017 Sep 6;16:524-538. doi: 10.1016/j.nicl.2017.09.005. eCollection 2017. Neuroimage Clin. 2017. PMID: 28948141 Free PMC article.
-
Impaired neurovascular coupling to ictal epileptic activity and spreading depolarization in a patient with subarachnoid hemorrhage: possible link to blood-brain barrier dysfunction.Epilepsia. 2012 Nov;53 Suppl 6(0 6):22-30. doi: 10.1111/j.1528-1167.2012.03699.x. Epilepsia. 2012. PMID: 23134492 Free PMC article. Review.
-
The continuum of spreading depolarizations in acute cortical lesion development: Examining Leão's legacy.J Cereb Blood Flow Metab. 2017 May;37(5):1571-1594. doi: 10.1177/0271678X16654495. Epub 2016 Jan 1. J Cereb Blood Flow Metab. 2017. PMID: 27328690 Free PMC article. Review.
Cited by
-
An updated review for clinical and radiological predictors of acute intraparenchymal hematoma progression in cerebral contusion.Heliyon. 2024 Oct 30;10(21):e39907. doi: 10.1016/j.heliyon.2024.e39907. eCollection 2024 Nov 15. Heliyon. 2024. PMID: 39553672 Free PMC article. Review.
-
Oxyhemoglobin and Cerebral Blood Flow Transients Detect Infarction in Rat Focal Brain Ischemia.Neuroscience. 2023 Jan 15;509:132-144. doi: 10.1016/j.neuroscience.2022.11.028. Epub 2022 Nov 29. Neuroscience. 2023. PMID: 36460221 Free PMC article.
-
Spreading Depolarizations Suppress Hematoma Growth in Hyperacute Intracerebral Hemorrhage in Mice.Stroke. 2023 Oct;54(10):2640-2651. doi: 10.1161/STROKEAHA.123.042632. Epub 2023 Aug 23. Stroke. 2023. PMID: 37610105 Free PMC article.
-
Numerical Simulation of Concussive-Generated Cortical Spreading Depolarization to Optimize DC-EEG Electrode Spacing for Noninvasive Visual Detection.Neurocrit Care. 2022 Jun;37(Suppl 1):67-82. doi: 10.1007/s12028-021-01430-x. Epub 2022 Mar 1. Neurocrit Care. 2022. PMID: 35233716 Free PMC article.
-
Cortical spreading depression: culprits and mechanisms.Exp Brain Res. 2022 Mar;240(3):733-749. doi: 10.1007/s00221-022-06307-9. Epub 2022 Jan 22. Exp Brain Res. 2022. PMID: 35064796 Review.
References
-
- O'Donnell MJ, Xavier D, Liu L, et al.. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet 2010; 376: 112–123. - PubMed
-
- van Asch CJ, Luitse MJ, Rinkel GJ, et al.. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol 2010; 9: 167–176. - PubMed
-
- Poon MT, Fonville AF, Al-Shahi Salman R.Long-term prognosis after intracerebral haemorrhage: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2014; 85: 660–667. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

