Randomized elimination and prolongation of ACE inhibitors and ARBs in coronavirus 2019 (REPLACE COVID) Trial Protocol
- PMID: 32937008
- PMCID: PMC7722152
- DOI: 10.1111/jch.14011
Randomized elimination and prolongation of ACE inhibitors and ARBs in coronavirus 2019 (REPLACE COVID) Trial Protocol
Abstract
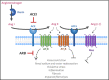
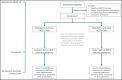
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus responsible for coronavirus disease 2019 (COVID-19), is associated with high incidence of multiorgan dysfunction and death. Angiotensin-converting enzyme 2 (ACE2), which facilitates SARS-CoV-2 host cell entry, may be impacted by angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs), two commonly used antihypertensive classes. In a multicenter, international randomized controlled trial that began enrollment on March 31, 2020, participants are randomized to continuation vs withdrawal of their long-term outpatient ACEI or ARB upon hospitalization with COVID-19. The primary outcome is a hierarchical global rank score incorporating time to death, duration of mechanical ventilation, duration of renal replacement or vasopressor therapy, and multiorgan dysfunction severity. Approval for the study has been obtained from the Institutional Review Board of each participating institution, and all participants will provide informed consent. A data safety monitoring board has been assembled to provide independent oversight of the project.
Keywords: COVID-19; angiotensin receptor blocker; angiotensin-converting enzyme inhibitor; angiotensin-converting enzyme inhibitor 2; clinical trial; coronavirus; hypertension.
© 2020 Wiley Periodicals LLC.
Conflict of interest statement
In the last 2 years, JAC has received consulting honoraria from Sanifit, Bristol Myers Squibb, Edwards Lifesciences, Bayer, and JNJ and research grants from the National Institutes of Health, Microsoft, Fukuda‐Denshi, and Bristol Myers Squibb. He has received compensation from the American Heart Association and the American College of Cardiology for editorial roles, and visiting speaker honoraria from Washington University and University of Utah. JS has received speaker honoraria and is on the advisory boards for AstraZeneca, Vifor Pharma, and Novo Nordisk, and has received speaker honoraria from AMGEN. JBB has received research grants from Fast Grants for this study, as well as from the National Institutes of Health. TIC has received funding paid by Janssen Pharmaceuticals to Stanford University; has served as a consultant for Bayer, Janssen Pharmaceuticals, Novo Nordisk, Fresenius Medical Care, Tricida, Gilead, and AstraZeneca; and has received grant support from Satellite Healthcare, the American Heart Association, and the National Institutes of Health.
Figures

References
-
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for disease control and prevention. JAMA. 2020;323(13):1239. - PubMed
-
- Sparks MA, Hiremath S, South A, et al. "The Coronavirus Conundrum: ACE2 and Hypertension Edition” NephJC. http://www.nephjc.com/news/covidace2. Accessed April 13, 2020.
Publication types
MeSH terms
Substances
Grants and funding
- T32-HL007891/NH/NIH HHS/United States
- K23 HL133843/HL/NHLBI NIH HHS/United States
- R61-HL-146390/NH/NIH HHS/United States
- R03 HL146874/HL/NHLBI NIH HHS/United States
- T32-DK07785/NH/NIH HHS/United States
- R01 HL121510/HL/NHLBI NIH HHS/United States
- R01 AG058969/AG/NIA NIH HHS/United States
- R56-HL136730/NH/NIH HHS/United States
- R00-HL141678/NH/NIH HHS/United States
- 1R01-HL104106/NH/NIH HHS/United States
- R00 HL141678/HL/NHLBI NIH HHS/United States
- P01-HL094307/NH/NIH HHS/United States
- P01 HL094307/HL/NHLBI NIH HHS/United States
- T32 DK007785/DK/NIDDK NIH HHS/United States
- R01-HL 121510-01A1/NH/NIH HHS/United States
- R01 HL104106/HL/NHLBI NIH HHS/United States
- R56 HL136730/HL/NHLBI NIH HHS/United States
- K23-HL133843/NH/NIH HHS/United States
- T32 HL007891/HL/NHLBI NIH HHS/United States
- R01 HL153646/HL/NHLBI NIH HHS/United States
- K99 HL141678/HL/NHLBI NIH HHS/United States
- R03-HL146874-01/NH/NIH HHS/United States
- R01-AG058969/NH/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

