Central Vestibular Tuning Arises from Patterned Convergence of Otolith Afferents
- PMID: 32937099
- PMCID: PMC7704800
- DOI: 10.1016/j.neuron.2020.08.019
Central Vestibular Tuning Arises from Patterned Convergence of Otolith Afferents
Erratum in
-
Central vestibular tuning arises from patterned convergence of otolith afferents.Neuron. 2021 Mar 3;109(5):905. doi: 10.1016/j.neuron.2021.02.005. Neuron. 2021. PMID: 33662271 Free PMC article. No abstract available.
Abstract
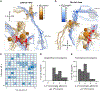
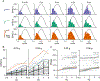
As sensory information moves through the brain, higher-order areas exhibit more complex tuning than lower areas. Though models predict that complexity arises via convergent inputs from neurons with diverse response properties, in most vertebrate systems, convergence has only been inferred rather than tested directly. Here, we measure sensory computations in zebrafish vestibular neurons across multiple axes in vivo. We establish that whole-cell physiological recordings reveal tuning of individual vestibular afferent inputs and their postsynaptic targets. Strong, sparse synaptic inputs can be distinguished by their amplitudes, permitting analysis of afferent convergence in vivo. An independent approach, serial-section electron microscopy, supports the inferred connectivity. We find that afferents with similar or differing preferred directions converge on central vestibular neurons, conferring more simple or complex tuning, respectively. Together, these results provide a direct, quantifiable demonstration of feedforward input convergence in vivo.
Keywords: body balance; electrical synapse; feedforward excitation; high-pass tuning; neural computation; sensorimotor transformation; sensory encoding; vestibulospinal neuron.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures

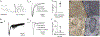
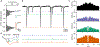



References
-
- ALONSO JM & MARTINEZ LM 1998. Functional connectivity between simple cells and complex cells in cat striate cortex. Nature Neuroscience, 1, 395–403. - PubMed
-
- ANGELAKI DE 1992. SPATIOTEMPORAL CONVERGENCE (STC) IN OTOLITH NEURONS. Biological Cybernetics, 67, 83–96. - PubMed
-
- ANGELAKI DE & CULLEN KE 2008. Vestibular system: The many facets of a multimodal sense. Annual Review of Neuroscience, 31, 125–150. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

