A Genetically Defined Compartmentalized Striatal Direct Pathway for Negative Reinforcement
- PMID: 32937106
- PMCID: PMC8605319
- DOI: 10.1016/j.cell.2020.08.032
A Genetically Defined Compartmentalized Striatal Direct Pathway for Negative Reinforcement
Abstract
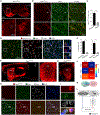
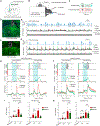
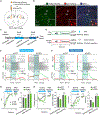
The striosome compartment within the dorsal striatum has been implicated in reinforcement learning and regulation of motivation, but how striosomal neurons contribute to these functions remains elusive. Here, we show that a genetically identified striosomal population, which expresses the Teashirt family zinc finger 1 (Tshz1) and belongs to the direct pathway, drives negative reinforcement and is essential for aversive learning in mice. Contrasting a "conventional" striosomal direct pathway, the Tshz1 neurons cause aversion, movement suppression, and negative reinforcement once activated, and they receive a distinct set of synaptic inputs. These neurons are predominantly excited by punishment rather than reward and represent the anticipation of punishment or the motivation for avoidance. Furthermore, inhibiting these neurons impairs punishment-based learning without affecting reward learning or movement. These results establish a major role of striosomal neurons in behaviors reinforced by punishment and moreover uncover functions of the direct pathway unaccounted for in classic models.
Keywords: Tshz1; aversive learning; avoidance; direct pathway; dorsal striatum; motivation; negative reinforcement; punishment; reward; striosome.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

