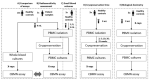
The Cytokinesis-Block Micronucleus Assay on Human Isolated Fresh and Cryopreserved Peripheral Blood Mononuclear Cells
- PMID: 32937746
- PMCID: PMC7564880
- DOI: 10.3390/jpm10030125
The Cytokinesis-Block Micronucleus Assay on Human Isolated Fresh and Cryopreserved Peripheral Blood Mononuclear Cells
Abstract
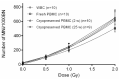
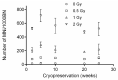
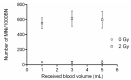
The cytokinesis-block micronucleus (CBMN) assay is a standardized method used for genotoxicity studies. Conventional whole blood cultures (WBC) are often used for this assay, although the assay can also be performed on isolated peripheral blood mononuclear cell (PBMC) cultures. However, the standardization of a protocol for the PBMC CBMN assay has not been investigated extensively. The aim of this study was to optimize a reliable CBMN assay protocol for fresh and cryopreserved peripheral blood mononuclear cells (PBMCS), and to compare micronuclei (MNi) results between WBC and PBMC cultures. The G0 CBMN assay was performed on whole blood, freshly isolated, and cryopreserved PBMCS from healthy human blood samples and five radiosensitive patient samples. Cells were exposed to 220 kV X-ray in vitro doses ranging from 0.5 to 2 Gy. The optimized PBMC CBMN assay showed adequate repeatability and small inter-individual variability. MNi values were significantly higher for WBC than for fresh PBMCS. Additionally, cryopreservation of PBMCS resulted in a significant increase of MNi values, while different cryopreservation times had no significant impact. In conclusion, our standardized CBMN assay on fresh and cryopreserved PBMCS can be used for genotoxicity studies, biological dosimetry, and radiosensitivity assessment.
Keywords: PBMCS; biological dosimetry; genotoxicity tests; human blood; micronucleus assay; radiosensitivity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Bonassi S., Znaor A., Ceppi M., Lando C., Chang W.P., Holland N., Kirsch-Volders M., Zeiger E., Ban S., Barale R., et al. An increased micronucleus frequency in peripheral blood lymphocytes predicts the risk of cancer in humans. Carcinogenesis. 2007;28:625–631. doi: 10.1093/carcin/bgl177. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

