Two Separate Tyrosine-Based YXXL/Φ Motifs within the Glycoprotein E Cytoplasmic Tail of Bovine Herpesvirus 1 Contribute in Virus Anterograde Neuronal Transport
- PMID: 32937797
- PMCID: PMC7551581
- DOI: 10.3390/v12091025
Two Separate Tyrosine-Based YXXL/Φ Motifs within the Glycoprotein E Cytoplasmic Tail of Bovine Herpesvirus 1 Contribute in Virus Anterograde Neuronal Transport
Abstract
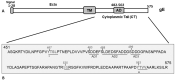
Bovine herpesvirus 1 (BHV-1) causes respiratory infection and abortion in cattle. Following a primary infection, BHV-1 establishes lifelong latency in the trigeminal ganglia (TG). Periodic reactivation of the latent virus in TG neurons results in anterograde virus transport to nerve endings in the nasal mucosa and nasal virus shedding. The BHV-1 glycoprotein E cytoplasmic tail (gE-CT) is necessary for virus cell-to-cell spread in epithelial cells and neuronal anterograde transport. Recently, we identified two tyrosine residues, Y467 and Y563, within the tyrosine-based motifs 467YTSL470 and 563YTVV566, which, together, account for the gE CT-mediated efficient cell-to-cell spread of BHV-1 in epithelial cells. Here, we determined that in primary neuron cultures in vitro, the individual alanine exchange Y467A or Y563A mutants had significantly diminished anterograde axonal spread. Remarkably, the double-alanine-exchanged Y467A/Y563A mutant virus was not transported anterogradely. Following intranasal infection of rabbits, both wild-type (wt) and the Y467A/Y563A mutant viruses established latency in the TG. Upon dexamethasone-induced reactivation, both wt and the mutant viruses reactivated and replicated equally efficiently in the TG. However, upon reactivation, only the wt, not the mutant, was isolated from nasal swabs. Therefore, the gE-CT tyrosine residues Y467 and Y563 together are required for gE CT-mediated anterograde neuronal transport.
Keywords: BHV-1; YXXL/Φ sorting motifs; anterograde neuronal traffic; compartmentalized primary neurons; gE CT domain; glycoprotein E; microfluidic chambers; reactivation from latency.
Conflict of interest statement
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
A Quadruple Gene-Deleted Live BoHV-1 Subunit RVFV Vaccine Vector Reactivates from Latency and Replicates in the TG Neurons of Calves but Is Not Transported to and Shed from Nasal Mucosa.Viruses. 2024 Sep 21;16(9):1497. doi: 10.3390/v16091497. Viruses. 2024. PMID: 39339973 Free PMC article.
-
A bovine herpesvirus type 1 mutant virus with truncated glycoprotein E cytoplasmic tail has defective anterograde neuronal transport in rabbit dorsal root ganglia primary neuronal cultures in a microfluidic chamber system.J Neurovirol. 2010 Nov;16(6):457-65. doi: 10.1007/BF03210851. Epub 2010 Nov 16. J Neurovirol. 2010. PMID: 21080783
-
A bovine herpesvirus type 1 mutant virus specifying a carboxyl-terminal truncation of glycoprotein E is defective in anterograde neuronal transport in rabbits and calves.J Virol. 2008 Aug;82(15):7432-42. doi: 10.1128/JVI.00379-08. Epub 2008 May 14. J Virol. 2008. PMID: 18480434 Free PMC article.
-
Regulation of the latency-reactivation cycle by products encoded by the bovine herpesvirus 1 (BHV-1) latency-related gene.J Neurovirol. 2011 Dec;17(6):535-45. doi: 10.1007/s13365-011-0060-3. Epub 2011 Dec 3. J Neurovirol. 2011. PMID: 22139602 Review.
-
A review of the biology of bovine herpesvirus type 1 (BHV-1), its role as a cofactor in the bovine respiratory disease complex and development of improved vaccines.Anim Health Res Rev. 2007 Dec;8(2):187-205. doi: 10.1017/S146625230700134X. Anim Health Res Rev. 2007. PMID: 18218160 Review.
Cited by
-
Recent Advances in the Study of Alphaherpesvirus Latency and Reactivation: Novel Guidance for the Design of Herpesvirus Live Vector Vaccines.Pathogens. 2024 Sep 10;13(9):779. doi: 10.3390/pathogens13090779. Pathogens. 2024. PMID: 39338969 Free PMC article. Review.
-
A Quadruple Gene-Deleted Live BoHV-1 Subunit RVFV Vaccine Vector Reactivates from Latency and Replicates in the TG Neurons of Calves but Is Not Transported to and Shed from Nasal Mucosa.Viruses. 2024 Sep 21;16(9):1497. doi: 10.3390/v16091497. Viruses. 2024. PMID: 39339973 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

