Beneficial Effects of Newly Isolated Akkermansia muciniphila Strains from the Human Gut on Obesity and Metabolic Dysregulation
- PMID: 32937828
- PMCID: PMC7564497
- DOI: 10.3390/microorganisms8091413
Beneficial Effects of Newly Isolated Akkermansia muciniphila Strains from the Human Gut on Obesity and Metabolic Dysregulation
Abstract
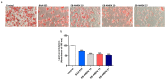
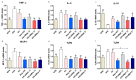
The identification of new probiotics with anti-obesity properties has attracted considerable interest. In the present study, the anti-obesity activities of Akkermansia muciniphila (A. muciniphila) strains isolated from human stool samples and their relationship with the gut microbiota were evaluated using a high fat-diet (HFD)-fed mice model. Three strains of A. muciniphila were chosen from 27 isolates selected based on their anti-lipogenic activity in 3T3-L1 cells. The anti-lipogenic, anti-adipogenic and anti-obesity properties of these three strains were evaluated further in HFD-induced obese mice. The animals were administered these strains six times per week for 12 weeks. The treatment improved the HFD-induced metabolic disorders in mice in terms of the prevention of body weight gain, caloric intake and reduction in the weights of the major adipose tissues and total fat. In addition, it improved glucose homeostasis and insulin sensitivity. These effects were also associated with the inhibition of low-grade intestinal inflammation and restoration of damaged gut integrity, prevention of liver steatosis and improvement of hepatic function. These results revealed a difference in the distribution pattern of the gut microbial communities between groups. Therefore, the gut microbial population modulation, at least in part, might contribute to the beneficial impact of the selected A. muciniphila strains against metabolic disorders.
Keywords: Akkermansia muciniphila; anti-obesity; gut microbiota; metabolic disorders; mice; probiotics.
Conflict of interest statement
Figures






References
-
- Puska P., Nishida C., Porter D., World Health Organization . Obesity and Overweight. World Health Organization; Geneve, Switzerland: 2003. pp. 1–2.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

