Mitochondrial Transfer by Human Mesenchymal Stromal Cells Ameliorates Hepatocyte Lipid Load in a Mouse Model of NASH
- PMID: 32937969
- PMCID: PMC7554948
- DOI: 10.3390/biomedicines8090350
Mitochondrial Transfer by Human Mesenchymal Stromal Cells Ameliorates Hepatocyte Lipid Load in a Mouse Model of NASH
Abstract
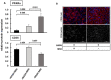
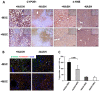
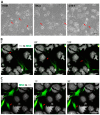
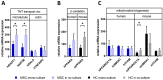
Mesenchymal stromal cell (MSC) transplantation ameliorated hepatic lipid load; tissue inflammation; and fibrosis in rodent animal models of non-alcoholic steatohepatitis (NASH) by as yet largely unknown mechanism(s). In a mouse model of NASH; we transplanted bone marrow-derived MSCs into the livers; which were analyzed one week thereafter. Combined metabolomic and proteomic data were applied to weighted gene correlation network analysis (WGCNA) and subsequent identification of key drivers. Livers were analyzed histologically and biochemically. The mechanisms of MSC action on hepatocyte lipid accumulation were studied in co-cultures of hepatocytes and MSCs by quantitative image analysis and immunocytochemistry. WGCNA and key driver analysis revealed that NASH caused the impairment of central carbon; amino acid; and lipid metabolism associated with mitochondrial and peroxisomal dysfunction; which was reversed by MSC treatment. MSC improved hepatic lipid metabolism and tissue homeostasis. In co-cultures of hepatocytes and MSCs; the decrease of lipid load was associated with the transfer of mitochondria from the MSCs to the hepatocytes via tunneling nanotubes (TNTs). Hence; MSCs may ameliorate lipid load and tissue perturbance by the donation of mitochondria to the hepatocytes. Thereby; they may provide oxidative capacity for lipid breakdown and thus promote recovery from NASH-induced metabolic impairment and tissue injury.
Keywords: mesenchymal stromal cells; non-alcoholic steatohepatitis (NASH); organelle transfer; primary hepatocytes; tunneling nanotubes (TNTs).
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources

