Treatment of Grade 3 and 4 Osteoarthritis with Intraoperatively Separated Adipose Tissue-Derived Stromal Vascular Fraction: A Comparative Case Series
- PMID: 32937996
- PMCID: PMC7565051
- DOI: 10.3390/cells9092096
Treatment of Grade 3 and 4 Osteoarthritis with Intraoperatively Separated Adipose Tissue-Derived Stromal Vascular Fraction: A Comparative Case Series
Abstract
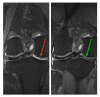
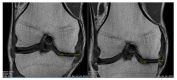
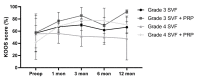
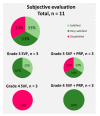
Osteoarthritis (OA) is the most common form of arthritis of the joints. The stromal vascular fraction (SVF) is a regenerative cell population that can be isolated from adipose tissue. It is the immunomodulatory properties of the stromal vascular fraction that make it a promising candidate for the regenerative treatment of OA. Patients with grade 3 and 4 osteoarthritis were treated with the stromal vascular fraction with and without platelet-rich plasma (PRP) and followed up on their Knee Injury and Osteoarthritis Outcome Score (KOOS) score for 12 months, with MRI and subjective evaluation of the procedure. Magnetic resonance imaging (MRI) revealed a widening of the joint space, a restructuring of the cartilage, and an alleviation of effusions in the treated joints. In three of the four treatment groups, a substantial improvement of the KOOS scores was documented at the 12-month follow-up time point. According to the subjective evaluation, 67% of the patients were satisfied or very satisfied with the procedure and would recommend it to others. No serious adverse events or unwanted side effects related to the SVF treatment were observed or reported. Prior to an invasive artificial joint replacement, the treatment of arthritic knee joints with the intraarticular injection of autologous adipose tissue-derived SVF should be considered a regenerative treatment option.
Keywords: Q-graft®; adipose tissue-derived stem/stromal cells (ASCs); osteoarthritis (OA); stromal vascular fraction (SVF); therapeutics.
Conflict of interest statement
Denis Simunec and Honey Salari have nothing to disclose. Juliane Meyer is an employee of Human Med AG. Products from this company were used in the study. All authors adhered to the Good Publication Practice guidelines for pharmaceutical companies.
Figures



References
-
- National Institute for Health and Care Excellence . Royal College of Physicians. National Institute for Health and Care Excellence; London, UK: 2014. Osteoarthritis Care and management in adults; pp. 1–505.
-
- Martel-Pelletier J., Barr A.J., Cicuttini F.M., Conaghan P.G., Cooper C., Goldring M.B., Goldring S.R., Jones G., Teichtahl A.J., Pelletier J.-P. Osteoarhritis. Nat. Rev. Dis. Prim. 2016;2:1–57. - PubMed
-
- Zhang W., Nuki G., Moskowitz R.W., Abramson S., Altman R.D., Arden N.K., Bierma-Zeinstra S., Brandt K.D., Croft P., Doherty M., et al. OARSI recommendations for the management of hip and knee osteoarthritis. Part III: Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthr. Cartil. 2010;18:476–499. doi: 10.1016/j.joca.2010.01.013. - DOI - PubMed
-
- Zhang W., Moskowitz R.W., Nuki G., Abramson S., Altman R.D., Arden N., Bierma-Zeinstra S., Brandt K.D., Croft P., Doherty M., et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthr. Cartil. 2008;16:137–162. doi: 10.1016/j.joca.2007.12.013. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

