Interviews with experts in rare diseases for the development of clinical decision support system software - a qualitative study
- PMID: 32938448
- PMCID: PMC7493382
- DOI: 10.1186/s12911-020-01254-3
Interviews with experts in rare diseases for the development of clinical decision support system software - a qualitative study
Abstract
Background: Patients with rare diseases (RDs) are often diagnosed too late or not at all. Clinical decision support systems (CDSSs) could support the diagnosis in RDs. The MIRACUM (Medical Informatics in Research and Medicine) consortium, which is one of four funded consortia in the German Medical Informatics Initiative, will develop a CDSS for RDs based on distributed clinical data from ten university hospitals. This qualitative study aims to investigate (1) the relevant organizational conditions for the operation of a CDSS for RDs when diagnose patients (e.g. the diagnosis workflow), (2) which data is necessary for decision support, and (3) the appropriate user group for such a CDSS.
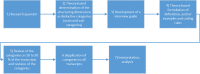
Methods: Interviews were carried out with RDs experts. Participants were recruited from staff physicians at the Rare Disease Centers (RDCs) at the MIRACUM locations, which offer diagnosis and treatment of RDs. An interview guide was developed with a category-guided deductive approach. The interviews were recorded on an audio device and then transcribed into written form. We continued data collection until all interviews were completed. Afterwards, data analysis was performed using Mayring's qualitative content analysis approach.
Results: A total of seven experts were included in the study. The results show that medical center guides and physicians from RDC B-centers (with a focus on different RDs) are involved in the diagnostic process. Furthermore, interdisciplinary case discussions between physicians are conducted. The experts explained that RDs exist which cannot be fully differentiated, but rather described only by their overall symptoms or findings: diagnosis is dependent on the disease or disease group. At the end of the diagnostic process, most centers prepare a summary of the patient case. Furthermore, the experts considered both physicians and experts from the B-centers to be potential users of a CDSS. The experts also have different experiences with CDSS for RDs.
Conclusions: This qualitative study is a first step towards establishing the requirements for the development of a CDSS for RDs. Further research is necessary to create solutions by also including the experts on RDs.
Keywords: Clinical decision support systems; Computer-assisted diagnosis; Interview; Qualitative research; Rare diseases.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Evaluation of a clinical decision support system for rare diseases: a qualitative study.BMC Med Inform Decis Mak. 2021 Feb 18;21(1):65. doi: 10.1186/s12911-021-01435-8. BMC Med Inform Decis Mak. 2021. PMID: 33602191 Free PMC article.
-
The Status Quo of Rare Diseases Centres for the Development of a Clinical Decision Support System - A Cross-Sectional Study.Stud Health Technol Inform. 2020 Jun 23;271:176-183. doi: 10.3233/SHTI200094. Stud Health Technol Inform. 2020. PMID: 32578561
-
Diagnosis of Rare Diseases: a scoping review of clinical decision support systems.Orphanet J Rare Dis. 2020 Sep 24;15(1):263. doi: 10.1186/s13023-020-01536-z. Orphanet J Rare Dis. 2020. PMID: 32972444 Free PMC article.
-
Clinicians' roles and necessary levels of understanding in the use of artificial intelligence: A qualitative interview study with German medical students.BMC Med Ethics. 2024 Oct 7;25(1):107. doi: 10.1186/s12910-024-01109-w. BMC Med Ethics. 2024. PMID: 39375660 Free PMC article.
-
Prescriber perceptions of medication-related computerized decision support systems in hospitals: A synthesis of qualitative research.Int J Med Inform. 2019 Sep;129:285-295. doi: 10.1016/j.ijmedinf.2019.06.024. Epub 2019 Jun 28. Int J Med Inform. 2019. PMID: 31445268 Review.
Cited by
-
Evaluating and Enhancing the Fitness-for-Purpose of Electronic Health Record Data: Qualitative Study on Current Practices and Pathway to an Automated Approach Within the Medical Informatics for Research and Care in University Medicine Consortium.JMIR Med Inform. 2024 Aug 19;12:e57153. doi: 10.2196/57153. JMIR Med Inform. 2024. PMID: 39158950 Free PMC article.
-
An intelligent decision support system for acute postoperative endophthalmitis: design, development and evaluation of a smartphone application.BMC Med Inform Decis Mak. 2023 Jul 21;23(1):130. doi: 10.1186/s12911-023-02214-3. BMC Med Inform Decis Mak. 2023. PMID: 37480036 Free PMC article.
-
Evaluation of a clinical decision support system for rare diseases: a qualitative study.BMC Med Inform Decis Mak. 2021 Feb 18;21(1):65. doi: 10.1186/s12911-021-01435-8. BMC Med Inform Decis Mak. 2021. PMID: 33602191 Free PMC article.
-
Documentation of hospitalization risk factors in electronic health records (EHRs): a qualitative study with home healthcare clinicians.J Am Med Inform Assoc. 2022 Apr 13;29(5):805-812. doi: 10.1093/jamia/ocac023. J Am Med Inform Assoc. 2022. PMID: 35196369 Free PMC article.
-
Financing of Rare Diseases and Orphan Drugs in A Sanctioned Country: A Qualitative Study.Med J Islam Repub Iran. 2022 May 11;36:48. doi: 10.47176/mjiri.36.48. eCollection 2022. Med J Islam Repub Iran. 2022. PMID: 36128263 Free PMC article.
References
-
- Knight A, Senior T. The common problem of rare disease in general practice. Med J Aust. 2006;185:82–83. - PubMed
-
- Taruscio D, Floridia G, Salvatore M, Groft SC, Gahl WA. Undiagnosed diseases: Italy-US collaboration and international efforts to tackle rare and common diseases lacking a diagnosis. Adv Exp Med Biol. 2017;1031:25–38. - PubMed
Publication types
MeSH terms
Grants and funding
- 01ZZ1801C/Bundesministerium für Bildung und Forschung/International
- 01ZZ1801A/Bundesministerium für Bildung und Forschung/International
- 01ZZ1801B/Bundesministerium für Bildung und Forschung/International
- 01ZZ1801C/Bundesministerium für Bildung und Forschung/International
- 01ZZ1801C/Bundesministerium für Bildung und Forschung/International
LinkOut - more resources
Full Text Sources
Medical