Synaptopodin Is Dispensable for Normal Podocyte Homeostasis but Is Protective in the Context of Acute Podocyte Injury
- PMID: 32938649
- PMCID: PMC7790210
- DOI: 10.1681/ASN.2020050572
Synaptopodin Is Dispensable for Normal Podocyte Homeostasis but Is Protective in the Context of Acute Podocyte Injury
Abstract
Background: Synaptopodin (Synpo) is an actin-associated protein in podocytes and dendritic spines. Many functions in regulating the actin cytoskeleton via RhoA and other pathways have been ascribed to Synpo, yet no pathogenic mutations in the SYNPO gene have been discovered in patients. Naturally occurring Synpo isoforms are known (Synpo-short and -long), and a novel truncated version (Synpo-T) is upregulated in podocytes from Synpo mutant mice. Synpo-T maintains some Synpo functions, which may prevent a podocyte phenotype from emerging in unchallenged mutant mice.
Methods: Novel mouse models were generated to further investigate the functions of Synpo. In one, CRISPR/Cas9 deleted most of the Synpo gene, preventing production of any detectable Synpo protein. Two other mutant strains made truncated versions of the protein. Adriamycin injections were used to challenge the mice, and Synpo functions were investigated in primary cultured podocytes.
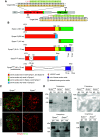
Results: Mice that could not make detectable Synpo (Synpo-/- ) did not develop any kidney abnormalities up to 12 months of age. However, Synpo-/- mice were more susceptible to Adriamycin nephropathy. In cultured primary podocytes from mutant mice, the absence of Synpo caused loss of stress fibers, increased the number and size of focal adhesions, and impaired cell migration. Furthermore, loss of Synpo led to decreased RhoA activity and increased Rac1 activation.
Conclusions: In contrast to previous findings, podocytes can function normally in vivo in the absence of any Synpo isoform. Synpo plays a protective role in the context of podocyte injury through its involvement in actin reorganization and focal adhesion dynamics.
Keywords: actin; cytoskeleton; glomerular filtration barrier; glomerulus podocyte.
Copyright © 2020 by the American Society of Nephrology.
Figures









References
-
- Kaplan JM, Kim SH, North KN, Rennke H, Correia LA, Tong HQ, et al. .: Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet 24: 251–256, 2000. - PubMed
-
- Kestilä M, Lenkkeri U, Männikkö M, Lamerdin J, McCready P, Putaala H, et al. .: Positionally cloned gene for a novel glomerular protein--nephrin--is mutated in congenital nephrotic syndrome. Mol Cell 1: 575–582, 1998. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

