T Cells from NOD- PerIg Mice Target Both Pancreatic and Neuronal Tissue
- PMID: 32938729
- PMCID: PMC7694871
- DOI: 10.4049/jimmunol.2000114
T Cells from NOD- PerIg Mice Target Both Pancreatic and Neuronal Tissue
Abstract
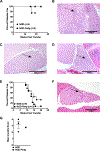
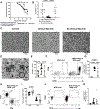
It has become increasingly appreciated that autoimmune responses against neuronal components play an important role in type 1 diabetes (T1D) pathogenesis. In fact, a large proportion of islet-infiltrating B lymphocytes in the NOD mouse model of T1D produce Abs directed against the neuronal type III intermediate filament protein peripherin. NOD-PerIg mice are a previously developed BCR-transgenic model in which virtually all B lymphocytes express the H and L chain Ig molecules from the intra-islet-derived anti-peripherin-reactive hybridoma H280. NOD-PerIg mice have accelerated T1D development, and PerIg B lymphocytes actively proliferate within islets and expand cognitively interactive pathogenic T cells from a pool of naive precursors. We now report adoptively transferred T cells or whole splenocytes from NOD-PerIg mice expectedly induce T1D in NOD.scid recipients but, depending on the kinetics of disease development, can also elicit a peripheral neuritis (with secondary myositis). This neuritis was predominantly composed of CD4+ and CD8+ T cells. Ab depletion studies showed neuritis still developed in the absence of NOD-PerIg CD8+ T cells but required CD4+ T cells. Surprisingly, sciatic nerve-infiltrating CD4+ cells had an expansion of IFN-γ- and TNF-α- double-negative cells compared with those within both islets and spleen. Nerve and islet-infiltrating CD4+ T cells also differed by expression patterns of CD95, PD-1, and Tim-3. Further studies found transitory early B lymphocyte depletion delayed T1D onset in a portion of NOD-PerIg mice, allowing them to survive long enough to develop neuritis outside of the transfer setting. Together, this study presents a new model of peripherin-reactive B lymphocyte-dependent autoimmune neuritis.
Copyright © 2020 by The American Association of Immunologists, Inc.
Figures




References
-
- Leiter E, and Atkinson M. 1998. NOD mice and related strains : research applications in diabetes, AIDS, cancer, and other diseases. R.G. Landes, Austin, Tex.
-
- Leiter EH 1993. The NOD Mouse: A Model for Analyzing the Interplay Between Heredity and Environment in Development of Autoimmune Disease. ILAR Journal 35: 4–14.
-
- Bour-Jordan H, Thompson HL, and Bluestone JA. 2005. Distinct effector mechanisms in the development of autoimmune neuropathy versus diabetes in nonobese diabetic mice. J Immunol 175: 5649–5655. - PubMed
-
- Louvet C, Kabre BG, Davini DW, Martinier N, Su MA, DeVoss JJ, Rosenthal WL, Anderson MS, Bour-Jordan H, and Bluestone JA. 2009. A novel myelin P0-specific T cell receptor transgenic mouse develops a fulminant autoimmune peripheral neuropathy. The Journal of experimental medicine 206: 507–514. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

