Diagnostic Approach for Arboviral Infections in the United States
- PMID: 32938736
- PMCID: PMC7685875
- DOI: 10.1128/JCM.01926-19
Diagnostic Approach for Arboviral Infections in the United States
Abstract
Domestic arthropod-borne viruses (arboviruses) are single-stranded RNA viruses, the most common of which include the mosquito-borne West Nile virus, St. Louis encephalitis virus, La Crosse virus, Jamestown Canyon virus, and eastern equine encephalitis virus, as well as the tick-borne Powassan virus. Previously considered rare infections, they have been detected with increasing frequency over the past 2 decades. Here, we present an overview of the domestic arboviruses listed above and describe the modalities employed to diagnose infection. Global arboviruses, including dengue virus, Zika virus, and chikungunya virus, have also been increasingly detected in the United States within the last 5 years but are not a focus of this minireview. Typical manifestations of arbovirus infection range from no symptoms, to meningitis or encephalitis, to death. Serologies are the standard means of diagnosis in the laboratory, since most viruses have a short period of replication, limiting the utility of molecular tests. The interpretation of serologies is confounded by antibody cross-reactivity with viruses belonging to the same serogroup and by long-lasting antibodies from prior infections. Next-generation assays have improved performance by increasing antigen purity, selecting optimal epitopes, and improving interpretive algorithms, but challenges remain. Due to cross-reactivity, a positive first-line serology test requires confirmation by either a plaque reduction neutralization test or detection of seroconversion or a 4-fold rise in virus-specific IgM or IgG antibody titers from acute- and convalescent-phase sera. The use of molecular diagnostics, such as reverse transcription PCR or unbiased metagenomic sequencing, is limited to the minority of patients who present with ongoing viremia or central nervous system replication. With the continued expansion of vector range, the diagnosis of domestic arboviruses will become an increasingly important task for generalists and specialists alike.
Keywords: Jamestown Canyon virus; La Crosse virus; Powassan virus; St. Louis encephalitis virus; West Nile virus; arbovirus; eastern equine encephalitis virus; encephalitis.
Copyright © 2020 American Society for Microbiology.
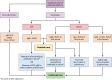
Figures

References
-
- Lindsey NP, Staples JE, Lehman JA, Fischer M, Centers for Disease Control and Prevention (CDC). 2010. Surveillance for human West Nile virus disease—United States, 1999–2008. MMWR Surveill Summ 59:1–17. - PubMed
-
- Piantadosi A, Rubin DB, McQuillen DP, Hsu L, Lederer PA, Ashbaugh CD, Duffalo C, Duncan R, Thon J, Bhattacharyya S, Basgoz N, Feske SK, Lyons JL. 2016. Emerging cases of Powassan virus encephalitis in New England: clinical presentation, imaging, and review of the literature. Clin Infect Dis 62:707–713. doi:10.1093/cid/civ1005. - DOI - PMC - PubMed
-
- Kinsella CM, Paras ML, Smole S, Mehta S, Ganesh V, Chen LH, McQuillen DP, Shah R, Chan J, Osborne M, Hennigan S, Halpern-Smith F, Brown CM, Sabeti P, Piantadosi A. 2020. Jamestown Canyon virus in Massachusetts: clinical case series and vector screening. Emerg Microbes Infect 9:903–912. doi:10.1080/22221751.2020.1756697. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

