Delayed Fulminant Hepatic Failure from Dydrogesterone-Related In Vitro Fertilization Therapy Requiring Liver Transplantation During Pregnancy
- PMID: 32938902
- PMCID: PMC7520868
- DOI: 10.12659/AJCR.925690
Delayed Fulminant Hepatic Failure from Dydrogesterone-Related In Vitro Fertilization Therapy Requiring Liver Transplantation During Pregnancy
Abstract
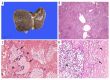
BACKGROUND Drug-induced liver failure is a rare complication of pregnancy and occasionally requires liver transplantation. However, fulminant liver failure arising from in vitro fertilization (IVF) therapy involving progestogens (e.g. dydrogesterone) is extremely rare and has not been reported in pregnancy. Furthermore, dydrogesterone-mediated hepatic dysfunction has not previously necessitated liver transplantation and is usually conservatively managed. We report the first Australian case of a pregnant woman with delayed fulminant liver failure and in utero fetal death requiring a liver transplant from dydrogesterone use. CASE REPORT A 35-year-old multiparous (G₅P₂) woman presented with painless jaundice and transaminitis (alanine aminotransferase and aspartate aminotransferase of 2800 U/L and 2990 U/L respectively). She was pregnant at 14 weeks' gestation after successful IVF in Thailand four months before involving dydrogesterone therapy. She was diagnosed with delayed, subfulminant liver failure arising from previous dydrogesterone use. Initially, she was not encephalopathic and conservative management strategies were instituted. Her hepatic dysfunction progressed and she deteriorated clinically with encephalopathy, necessitating an emergent liver transplantation. Fetal death was confirmed in utero four days before transplantation. A combined orthotopic liver transplant and hysterotomy with fetal evacuation were performed without complication. CONCLUSIONS Fulminant liver failure in pregnancy due to idiosyncratic drug reactions are rare. Dydrogesterone may cause significant, albeit delayed, liver dysfunction in pregnancy necessitating the need for liver transplantation. Early recognition of progressive liver failure despite best supportive care efforts should prompt early considerations for liver transplantation. Delays in liver transplantation with prolonged hyperbilirubinemia and coagulopathy may exacerbate fetal death in utero.
Conflict of interest statement
None.
Figures

References
-
- Nelson DB, Yost NP, Cunningham G. Acute fatty liver of pregnancy: Clinical outcomes and expected duration of recovery. Am J Obstet Gynecol. 2013;209:e1–7. - PubMed
-
- Russo MW, Galanko JA, Shrestha R, et al. Liver transplantation for acute liver failure from drug induced liver injury in the United States. Liver Transpl. 2004;10:1018–23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

