Deregulated immune cell recruitment orchestrated by FOXM1 impairs human diabetic wound healing
- PMID: 32938916
- PMCID: PMC7495445
- DOI: 10.1038/s41467-020-18276-0
Deregulated immune cell recruitment orchestrated by FOXM1 impairs human diabetic wound healing
Abstract
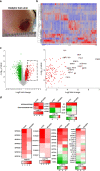
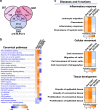
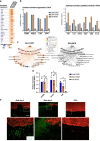
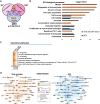
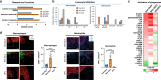
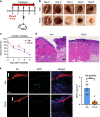
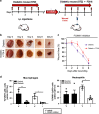
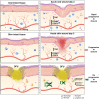
Diabetic foot ulcers (DFUs) are a life-threatening disease that often result in lower limb amputations and a shortened lifespan. However, molecular mechanisms contributing to the pathogenesis of DFUs remain poorly understood. We use next-generation sequencing to generate a human dataset of pathogenic DFUs to compare to transcriptional profiles of human skin and oral acute wounds, oral as a model of "ideal" adult tissue repair due to accelerated closure without scarring. Here we identify major transcriptional networks deregulated in DFUs that result in decreased neutrophils and macrophages recruitment and overall poorly controlled inflammatory response. Transcription factors FOXM1 and STAT3, which function to activate and promote survival of immune cells, are inhibited in DFUs. Moreover, inhibition of FOXM1 in diabetic mouse models (STZ-induced and db/db) results in delayed wound healing and decreased neutrophil and macrophage recruitment in diabetic wounds in vivo. Our data underscore the role of a perturbed, ineffective inflammatory response as a major contributor to the pathogenesis of DFUs, which is facilitated by FOXM1-mediated deregulation of recruitment of neutrophils and macrophages, revealing a potential therapeutic strategy.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
FOXM1 network in association with TREM1 suppression regulates NET formation in diabetic foot ulcers.EMBO Rep. 2022 Aug 3;23(8):e54558. doi: 10.15252/embr.202154558. Epub 2022 Jul 20. EMBO Rep. 2022. PMID: 35856334 Free PMC article.
-
Exploring the mechanism by which UCHL3 alleviates diabetic foot ulcers: FOXM1/NLRP3 inflammasome-mediated angiogenesis and endothelial cell pyroptosis.J Orthop Surg Res. 2025 May 20;20(1):488. doi: 10.1186/s13018-025-05914-w. J Orthop Surg Res. 2025. PMID: 40394598 Free PMC article.
-
Suppression of TRIM72-mediated endoplasmic reticulum stress facilitates FOXM1 promotion of diabetic ulcer healing.Wound Repair Regen. 2025 Jan-Feb;33(1):e13247. doi: 10.1111/wrr.13247. Wound Repair Regen. 2025. PMID: 39721954 Free PMC article.
-
Strategy for Treatment of Infected Diabetic Foot Ulcers.Acc Chem Res. 2021 Mar 2;54(5):1080-1093. doi: 10.1021/acs.accounts.0c00864. Epub 2021 Feb 17. Acc Chem Res. 2021. PMID: 33596041 Review.
-
Neuropeptides, Inflammation, Biofilms, and diabetic Foot Ulcers.Exp Clin Endocrinol Diabetes. 2022 Jul;130(7):439-446. doi: 10.1055/a-1493-0458. Epub 2021 Jul 5. Exp Clin Endocrinol Diabetes. 2022. PMID: 34225369 Review.
Cited by
-
Fibroblasts in Diabetic Foot Ulcers.Int J Mol Sci. 2024 Feb 11;25(4):2172. doi: 10.3390/ijms25042172. Int J Mol Sci. 2024. PMID: 38396848 Free PMC article. Review.
-
Mannose-Decorated Co-Polymer Facilitates Controlled Release of Butyrate to Accelerate Chronic Wound Healing.Adv Healthc Mater. 2023 Oct;12(26):e2300515. doi: 10.1002/adhm.202300515. Epub 2023 Aug 22. Adv Healthc Mater. 2023. PMID: 37503634 Free PMC article.
-
Exploration of the common pathogenic link between COVID-19 and diabetic foot ulcers: An in silico approach.Health Sci Rep. 2023 Nov 5;6(11):e1686. doi: 10.1002/hsr2.1686. eCollection 2023 Nov. Health Sci Rep. 2023. PMID: 37936615 Free PMC article.
-
Qualitative study on diabetic cutaneous wound healing with radiation crosslinked bilayer collagen scaffold in rat model.Sci Rep. 2023 Apr 19;13(1):6399. doi: 10.1038/s41598-023-33372-z. Sci Rep. 2023. PMID: 37076561 Free PMC article.
-
Exosomes from adipose-derived stem cells restore fibroblast function and accelerate diabetic wound healing.Heliyon. 2023 Nov 28;10(1):e22802. doi: 10.1016/j.heliyon.2023.e22802. eCollection 2024 Jan 15. Heliyon. 2023. PMID: 38163237 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

