Bacteria are important dimethylsulfoniopropionate producers in marine aphotic and high-pressure environments
- PMID: 32938931
- PMCID: PMC7494906
- DOI: 10.1038/s41467-020-18434-4
Bacteria are important dimethylsulfoniopropionate producers in marine aphotic and high-pressure environments
Abstract
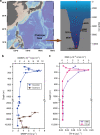
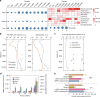
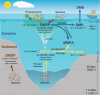
Dimethylsulfoniopropionate (DMSP) is an important marine osmolyte. Aphotic environments are only recently being considered as potential contributors to global DMSP production. Here, our Mariana Trench study reveals a typical seawater DMSP/dimethylsulfide (DMS) profile, with highest concentrations in the euphotic zone and decreased but consistent levels below. The genetic potential for bacterial DMSP synthesis via the dsyB gene and its transcription is greater in the deep ocean, and is highest in the sediment.s DMSP catabolic potential is present throughout the trench waters, but is less prominent below 8000 m, perhaps indicating a preference to store DMSP in the deep for stress protection. Deep ocean bacterial isolates show enhanced DMSP production under increased hydrostatic pressure. Furthermore, bacterial dsyB mutants are less tolerant of deep ocean pressures than wild-type strains. Thus, we propose a physiological function for DMSP in hydrostatic pressure protection, and that bacteria are key DMSP producers in deep seawater and sediment.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Ksionzek KB, et al. Dissolved organic sulfur in the ocean: Biogeochemistry of a petagram inventory. Science. 2016;354:456–459. - PubMed
-
- Zhang XH, et al. Biogenic production of DMSP and its degradation to DMS—their roles in the global sulfur cycle. Sci. China Life Sci. 2019;62:1296–1319. - PubMed
-
- Sunda W, Kieber DJ, Kiene RP, Huntsman S. An antioxidant function for DMSP and DMS in marine algae. Nature. 2002;418:317–320. - PubMed
-
- Kiene RP, Linn LJ, Bruton JA. New and important roles for DMSP in marine microbial communities. J. Sea Res. 2000;43:209–224.
-
- Strom S, Wolfe G, Slajer A, Lambert S, Clough J. Chemical defense in the microplankton II: inhibition of protist feeding by beta-dimethylsulfoniopropionate (DMSP) Limnol. Oceanogr. 2003;48:230–237.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

