Hemoglobin stimulates vigorous growth of Streptococcus pneumoniae and shapes the pathogen's global transcriptome
- PMID: 32938947
- PMCID: PMC7494912
- DOI: 10.1038/s41598-020-71910-1
Hemoglobin stimulates vigorous growth of Streptococcus pneumoniae and shapes the pathogen's global transcriptome
Abstract
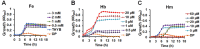
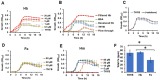
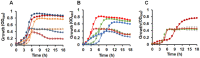
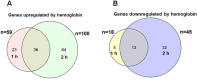
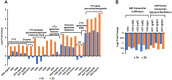
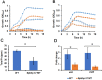
Streptococcus pneumoniae (Spn) must acquire iron from the host to establish infection. We examined the impact of hemoglobin, the largest iron reservoir in the body, on pneumococcal physiology. Supplementation with hemoglobin allowed Spn to resume growth in an iron-deplete medium. Pneumococcal growth with hemoglobin was unusually robust, exhibiting a prolonged logarithmic growth, higher biomass, and extended viability in both iron-deplete and standard medium. We observed the hemoglobin-dependent response in multiple serotypes, but not with other host proteins, free iron, or heme. Remarkably, hemoglobin induced a sizable transcriptome remodeling, effecting virulence and metabolism in particular genes facilitating host glycoconjugates use. Accordingly, Spn was more adapted to grow on the human α - 1 acid glycoprotein as a sugar source with hemoglobin. A mutant in the hemoglobin/heme-binding protein Spbhp-37 was impaired for growth on heme and hemoglobin iron. The mutant exhibited reduced growth and iron content when grown in THYB and hemoglobin. In summary, the data show that hemoglobin is highly beneficial for Spn cultivation in vitro and suggest that hemoglobin might drive the pathogen adaptation in vivo. The hemoglobin receptor, Spbhp-37, plays a role in mediating the positive influence of hemoglobin. These novel findings provide intriguing insights into pneumococcal interactions with its obligate human host.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Musher DM, Rueda AM, Kaka AS, Mapara SM. The association between pneumococcal pneumonia and acute cardiac events. Clin. Infect. Dis. 2007;45:158–165. - PubMed
-
- Kadioglu A, Weiser JN, Paton JC, Andrew PW. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat. Rev. Microbiol. 2008;6:288–301. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

