An engineered antibody binds a distinct epitope and is a potent inhibitor of murine and human VISTA
- PMID: 32938950
- PMCID: PMC7494997
- DOI: 10.1038/s41598-020-71519-4
An engineered antibody binds a distinct epitope and is a potent inhibitor of murine and human VISTA
Abstract
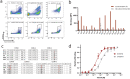
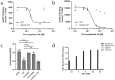
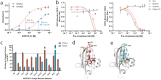
V-domain immunoglobulin (Ig) suppressor of T cell activation (VISTA) is an immune checkpoint that maintains peripheral T cell quiescence and inhibits anti-tumor immune responses. VISTA functions by dampening the interaction between myeloid cells and T cells, orthogonal to PD-1 and other checkpoints of the tumor-T cell signaling axis. Here, we report the use of yeast surface display to engineer an anti-VISTA antibody that binds with high affinity to mouse, human, and cynomolgus monkey VISTA. Our anti-VISTA antibody (SG7) inhibits VISTA function and blocks purported interactions with both PSGL-1 and VSIG3 proteins. SG7 binds a unique epitope on the surface of VISTA, which partially overlaps with other clinically relevant antibodies. As a monotherapy, and to a greater extent as a combination with anti-PD1, SG7 slows tumor growth in multiple syngeneic mouse models. SG7 is a promising clinical candidate that can be tested in fully immunocompetent mouse models and its binding epitope can be used for future campaigns to develop species cross-reactive inhibitors of VISTA.
Conflict of interest statement
N.M., S.M., R.K, and J.R.C. are included as inventors on intellectual property related to the work described in this manuscript. J.R.C. is a co-founder and J.R.C and R.K. are shareholders in xCella Biosciences, which is developing antibody therapeutics for applications in oncology. Other authors declare no competing financial interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

