Mechanism for the reactivation of the peroxidase activity of human cyclooxygenases: investigation using phenol as a reducing cosubstrate
- PMID: 32938962
- PMCID: PMC7494923
- DOI: 10.1038/s41598-020-71237-x
Mechanism for the reactivation of the peroxidase activity of human cyclooxygenases: investigation using phenol as a reducing cosubstrate
Abstract
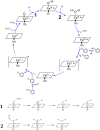
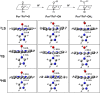
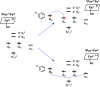
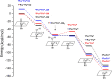
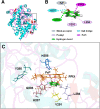
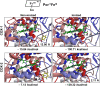
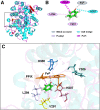
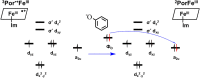
It has been known for many years that the peroxidase activity of cyclooxygenase 1 and 2 (COX-1 and COX-2) can be reactivated in vitro by the presence of phenol, which serves as a reducing compound, but the underlying mechanism is still poorly understood. In the present study, we use phenol as a model compound to investigate the mechanism by which the peroxidase activity of human COXs is reactivated after each catalytic cycle. Molecular docking and quantum mechanics calculations are carried out to probe the interaction of phenol with the peroxidase site of COXs and the reactivation mechanism. It is found that the oxygen atom associated with the Fe ion in the heme group (i.e., the complex of Fe ion and porphyrin) of COXs can be removed by addition of two protons. Following its removal, phenol can readily bind inside the peroxidase active sites of the COX enzymes, and directly interact with Fe in heme to facilitate electron transfer from phenol to heme. This investigation provides theoretical evidence for several intermediates formed in the COX peroxidase reactivation cycle, thereby unveiling mechanistic details that would aid in future rational design of drugs that target the peroxidase site.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Mechanism of reactivation of the peroxidase catalytic activity of human cyclooxygenases by reducing cosubstrate quercetin.J Mol Graph Model. 2021 Sep;107:107941. doi: 10.1016/j.jmgm.2021.107941. Epub 2021 May 28. J Mol Graph Model. 2021. PMID: 34091174
-
Substitution of tyrosine for the proximal histidine ligand to the heme of prostaglandin endoperoxide synthase 2: implications for the mechanism of cyclooxygenase activation and catalysis.Biochemistry. 2000 May 9;39(18):5422-32. doi: 10.1021/bi992752f. Biochemistry. 2000. PMID: 10820014
-
The structure of mammalian cyclooxygenases.Annu Rev Biophys Biomol Struct. 2003;32:183-206. doi: 10.1146/annurev.biophys.32.110601.141906. Epub 2003 Feb 5. Annu Rev Biophys Biomol Struct. 2003. PMID: 12574066 Review.
-
Mutational analysis of the role of the distal histidine and glutamine residues of prostaglandin-endoperoxide synthase-2 in peroxidase catalysis, hydroperoxide reduction, and cyclooxygenase activation.J Biol Chem. 1997 Aug 22;272(34):21565-74. doi: 10.1074/jbc.272.34.21565. J Biol Chem. 1997. PMID: 9261177
-
Mechanism of free radical oxygenation of polyunsaturated fatty acids by cyclooxygenases.Chem Rev. 2003 Jun;103(6):2239-304. doi: 10.1021/cr000068x. Chem Rev. 2003. PMID: 12797830 Review. No abstract available.
Cited by
-
2-Hydroxybenzohydrazide as a novel potential candidate against nociception, inflammation, and pyrexia: in vitro, in vivo, and computational approaches.Inflammopharmacology. 2024 Feb;32(1):643-656. doi: 10.1007/s10787-023-01356-0. Epub 2023 Oct 21. Inflammopharmacology. 2024. PMID: 37864684
-
Ellagic acid, a plant phenolic compound, activates cyclooxygenase-mediated prostaglandin production.Exp Ther Med. 2019 Aug;18(2):987-996. doi: 10.3892/etm.2019.7667. Epub 2019 Jun 13. Exp Ther Med. 2019. PMID: 31316596 Free PMC article.
-
Globularia alypum L. and Related Species: LC-MS Profiles and Antidiabetic, Antioxidant, Anti-Inflammatory, Antibacterial and Anticancer Potential.Pharmaceuticals (Basel). 2022 Apr 21;15(5):506. doi: 10.3390/ph15050506. Pharmaceuticals (Basel). 2022. PMID: 35631332 Free PMC article.
References
-
- Hamberg M, Samuelsson B. Oxygenation of unsaturated fatty acids by the vesicular gland of sheep. J. Biol. Chem. 1967;242:5344–5354. - PubMed
-
- Miyamoto T, Ogino N, Yamamoto S, Hayaishi O. Purification of prostaglandin endoperoxide synthetase from bovine vesicular gland microsomes. J. Biol. Chem. 1976;251:2629–2636. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

