Attenuating hypoxia driven malignant behavior in glioblastoma with a novel hypoxia-inducible factor 2 alpha inhibitor
- PMID: 32938997
- PMCID: PMC7495485
- DOI: 10.1038/s41598-020-72290-2
Attenuating hypoxia driven malignant behavior in glioblastoma with a novel hypoxia-inducible factor 2 alpha inhibitor
Abstract
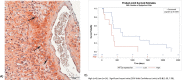
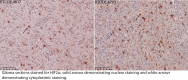
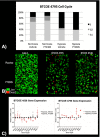
Hypoxia inducible factor (HIFs) signaling contributes to malignant cell behavior in glioblastoma (GBM). We investigated a novel HIF2α inhibitor, PT2385, both in vitro, with low-passage patient-derived cell lines, and in vivo, using orthotopic models of glioblastoma. We focused on analysis of HIF2α expression in situ, cell survival/proliferation, and survival in brain tumor-bearing mice treated with PT2385 alone and in combination with standard of care chemoradiotherapy. HIF2α expression increased with glioma grade, with over half of GBM specimens HIF2α positive. Staining clustered in perivascular and perinecrotic tumor regions. Cellular phenotype including proliferation, viability, migration/invasion, and also gene expression were not altered after PT2385 treatment. In the animal model, PT2385 single-agent treatment did improve median overall survival compared to placebo (p = 0.04, n = 21) without a bioluminescence correlate (t = 0.67, p = 0.52). No difference in animal survival was seen in combination treatment with radiation (RT)/temozolomide (TMZ)/PT2385 (p = 0.44, n = 10) or mean tumor bioluminescence (t 1.13, p = 0.32). We conclude that HIF2α is a reasonable novel therapeutic target as expressed in the majority of glioblastomas in our cohort. PT2385 as a single-agent was efficacious in vivo, however, an increase in animal survival was not seen with PT2385 in combination with RT/TMZ. Further study for targeting HIF2α as a therapeutic approach in GBM is warranted.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
ARNT-dependent HIF-2 transcriptional activity is not sufficient to regulate downstream target genes in neuroblastoma.Exp Cell Res. 2020 Mar 15;388(2):111845. doi: 10.1016/j.yexcr.2020.111845. Epub 2020 Jan 13. Exp Cell Res. 2020. PMID: 31945318
-
Differential effects of HIF2α antagonist and HIF2α silencing in renal cancer and sensitivity to repurposed drugs.BMC Cancer. 2021 Aug 5;21(1):896. doi: 10.1186/s12885-021-08616-8. BMC Cancer. 2021. PMID: 34353313 Free PMC article.
-
Hypoxia-inducible factor 2α: a novel target in gliomas.Future Med Chem. 2018 Sep 1;10(18):2227-2236. doi: 10.4155/fmc-2018-0163. Epub 2018 Aug 9. Future Med Chem. 2018. PMID: 30089425 Free PMC article. Review.
-
Targeting HIF-2α in glioblastoma reshapes the immune infiltrate and enhances response to immune checkpoint blockade.Cell Mol Life Sci. 2025 Mar 17;82(1):119. doi: 10.1007/s00018-025-05642-8. Cell Mol Life Sci. 2025. PMID: 40095115 Free PMC article.
-
Targeting HIF-2 α in clear cell renal cell carcinoma: A promising therapeutic strategy.Crit Rev Oncol Hematol. 2017 Mar;111:117-123. doi: 10.1016/j.critrevonc.2017.01.013. Epub 2017 Jan 28. Crit Rev Oncol Hematol. 2017. PMID: 28259286 Review.
Cited by
-
Increased Ascorbate Content of Glioblastoma Is Associated With a Suppressed Hypoxic Response and Improved Patient Survival.Front Oncol. 2022 Mar 28;12:829524. doi: 10.3389/fonc.2022.829524. eCollection 2022. Front Oncol. 2022. PMID: 35419292 Free PMC article.
-
Interactions Between Anti-Angiogenic Therapy and Immunotherapy in Glioblastoma.Front Oncol. 2022 Jan 12;11:812916. doi: 10.3389/fonc.2021.812916. eCollection 2021. Front Oncol. 2022. PMID: 35096619 Free PMC article. Review.
-
Expression of STAT3 and hypoxia markers in long-term surviving malignant glioma patients.BMC Cancer. 2024 Apr 23;24(1):509. doi: 10.1186/s12885-024-12221-w. BMC Cancer. 2024. PMID: 38654280 Free PMC article.
-
Molecular Targeted Therapies in Glioblastoma Multiforme: A Systematic Overview of Global Trends and Findings.Brain Sci. 2023 Nov 17;13(11):1602. doi: 10.3390/brainsci13111602. Brain Sci. 2023. PMID: 38002561 Free PMC article. Review.
-
Targeting HIF-2α in the Tumor Microenvironment: Redefining the Role of HIF-2α for Solid Cancer Therapy.Cancers (Basel). 2022 Feb 28;14(5):1259. doi: 10.3390/cancers14051259. Cancers (Basel). 2022. PMID: 35267567 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

