S-Adenosylmethionine (SAMe) in major depressive disorder (MDD): a clinician-oriented systematic review
- PMID: 32939220
- PMCID: PMC7487540
- DOI: 10.1186/s12991-020-00298-z
S-Adenosylmethionine (SAMe) in major depressive disorder (MDD): a clinician-oriented systematic review
Abstract
Background: Major depressive disorder (MDD) is a recurrent illness with high rates of chronicity, treatment-resistance, and significant economic impact. S-Adenosylmethionine (SAMe), a molecule that is formed naturally in the human body, has shown antidepressant effects and may expand the available options for treating MDD. This systematic review examines the evidence concerning the efficacy of SAMe as monotherapy or in combination with antidepressants.
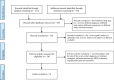
Methods: A systematic search in Medline, Psychinfo, AMED, and Cochrane Controlled Trials Register was conducted for any reference recorded up to March 2020. Double-blind, randomised controlled trials, comparing the antidepressant efficacy of SAMe to placebo or/and to other antidepressants, were selected. Two authors evaluated each study independently and then, reconciled findings.
Results: Eight trials, with a total of 11 arms and 1011 subjects, evaluating the efficacy of SAMe used as monotherapy or as adjunctive therapy (512 individuals), were included in this review. The study duration ranged between 2 and 12 weeks and the daily dose of SAMe varied from 200 to 3200 mg. Five comparisons evaluated the differences between SAMe and placebo and SAMe resulted significantly better than placebo in three of these studies. Four comparisons evaluated the differences between SAMe and other antidepressants (imipramine or escitalopram) and showed no significant difference. One study showed that SAMe was significantly better than placebo in accelerating the response to imipramine from day 4 to day 12, but the mean scores were not statistically different at the day 14 endpoint. One study showed that SAMe combined with serotonin reuptake inhibitors (SSRI) was better than PBO combined with SSRI. The studies reported only mild, transient or non-clinically relevant side effects.
Conclusions: The existing trials of SAMe, used as monotherapy or add on to another antidepressants, have shown encouraging and generally positive results. However, more evidence is necessary before definitive conclusions can be drawn. Larger, double-blind randomised controlled studies are warranted to confirm the antidepressant effectiveness of SAMe.
Keywords: Antidepressant; Depression; Nutraceutical; S-Adenosylmethionine; Systematic review.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsAndrea Fagiolini is/has been a consultant and/or a speaker and/or has received research grants from Allergan, Angelini, Apsen, Boehringer Ingelheim, Doc Generici, FB-Health, Italfarmaco, Janssen, Lundbeck, Mylan, Otsuka, Pfizer, Recordati, Sanofi Aventis, Sunovion, Vifor. Alessandro Cuomo is/has been a consultant and/or a speaker and/or has received research grants from Angelini, Janssen, Lundbeck, Otsuka, and Pfizer. The other authors declare no conflict of interest.
Figures
References
-
- World Health Organization. Depression 30’, Fact Sheet, 2020. https://www.who.int/news-room/fact-sheets/detail/depression. Accessed 8 Jan 2020.
Publication types
LinkOut - more resources
Full Text Sources