Actin reorganization at the centrosomal area and the immune synapse regulates polarized secretory traffic of multivesicular bodies in T lymphocytes
- PMID: 32939232
- PMCID: PMC7480611
- DOI: 10.1080/20013078.2020.1759926
Actin reorganization at the centrosomal area and the immune synapse regulates polarized secretory traffic of multivesicular bodies in T lymphocytes
Abstract
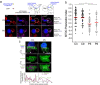
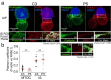
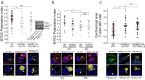
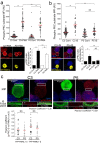
T-cell receptor stimulation induces the convergence of multivesicular bodies towards the microtubule-organizing centre (MTOC) and the polarization of the MTOC to the immune synapse (IS). These events lead to exosome secretion at the IS. We describe here that upon IS formation centrosomal area F-actin decreased concomitantly with MTOC polarization to the IS. PKCδ-interfered T cell clones showed a sustained level of centrosomal area F-actin associated with defective MTOC polarization. We analysed the contribution of two actin cytoskeleton-regulatory proteins, FMNL1 and paxillin, to the regulation of cortical and centrosomal F-actin networks. FMNL1 phosphorylation and F-actin reorganization at the IS were inhibited in PKCδ-interfered clones. F-actin depletion at the central region of the IS, a requirement for MTOC polarization, was associated with FMNL1 phosphorylation at its C-terminal, autoregulatory region. Interfering all FMNL1 isoforms prevented MTOC polarization; nonetheless, FMNL1 re-expression restored MTOC polarization in a centrosomal area F-actin reorganization-independent manner. Moreover, PKCδ-interfered clones exhibited decreased paxillin phosphorylation at the MTOC, which suggests an alternative actin cytoskeleton regulatory pathway. Our results infer that PKCδ regulates MTOC polarization and secretory traffic leading to exosome secretion in a coordinated manner by means of two distinct pathways, one involving FMNL1 regulation and controlling F-actin reorganization at the IS, and the other, comprising paxillin phosphorylation potentially controlling centrosomal area F-actin reorganization.
Abbreviations: Ab, antibody; AICD, activation-induced cell death; AIP, average intensity projection; APC, antigen-presenting cell; BCR, B-cell receptor for antigen; C, centre of mass; cent2, centrin 2; cIS, central region of the immune synapse; CMAC, CellTracker™ Blue (7-amino-4-chloromethylcoumarin); cSMAC, central supramolecular activation cluster; CTL, cytotoxic T lymphocytes; DAG, diacylglycerol; DGKα, diacylglycerol kinase α; Dia1, Diaphanous-1; dSMAC, distal supramolecular activation cluster; ECL, enhanced chemiluminescence; ESCRT, endosomal sorting complex required for traffic; F-actin, filamentous actin; Fact-low cIS, F-actin-low region at the centre of the immune synapse; FasL, Fas ligand; FMNL1, formin-like 1; fps, frames per second; GFP, green fluorescent protein; HBSS, Hank's balanced salt solution; HRP, horseradish peroxidase; ILV, intraluminal vesicles; IS, immune synapse; MFI, mean fluorescence intensity; MHC, major histocompatibility complex; MIP, maximal intensity projection; MVB, multivesicular bodies; MTOC, microtubule-organizing centre; NS, not significant; PBL, peripheral blood lymphocytes; PKC, protein kinase C; PKCδ, protein kinase C δ isoform; PLC, phospholipase C; PMA, phorbol myristate acetate; Pol. Index, polarization index; pSMAC, peripheral supramolecular activation cluster; PSF, point spread function; ROI, region of interest; SD, standard deviation; shRNA, short hairpin RNA; SEE, Staphylococcus enterotoxin E; SMAC, supramolecular activation cluster; TCR, T-cell receptor for antigen; T-helper (Th); TRANS, transmittance; WB, Western blot.
Keywords: FMNL1; T lymphocytes; actin cytoskeleton; centrosome; immune synapse; multivesicular bodies; paxillin; protein kinase C δ.
© 2020 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group on behalf of The International Society for Extracellular Vesicles.
Conflict of interest statement
The authors report no conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

