Machine learning deciphers structural features of RNA duplexes measured with solution X-ray scattering
- PMID: 32939279
- PMCID: PMC7467162
- DOI: 10.1107/S2052252520008830
Machine learning deciphers structural features of RNA duplexes measured with solution X-ray scattering
Abstract
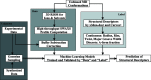
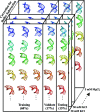
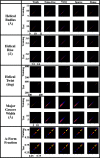
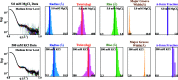
Macromolecular structures can be determined from solution X-ray scattering. Small-angle X-ray scattering (SAXS) provides global structural information on length scales of 10s to 100s of Ångstroms, and many algorithms are available to convert SAXS data into low-resolution structural envelopes. Extension of measurements to wider scattering angles (WAXS or wide-angle X-ray scattering) can sharpen the resolution to below 10 Å, filling in structural details that can be critical for biological function. These WAXS profiles are especially challenging to interpret because of the significant contribution of solvent in addition to solute on these smaller length scales. Based on training with molecular dynamics generated models, the application of extreme gradient boosting (XGBoost) is discussed, which is a supervised machine learning (ML) approach to interpret features in solution scattering profiles. These ML methods are applied to predict key structural parameters of double-stranded ribonucleic acid (dsRNA) duplexes. Duplex conformations vary with salt and sequence and directly impact the foldability of functional RNA molecules. The strong structural periodicities in these duplexes yield scattering profiles with rich sets of features at intermediate-to-wide scattering angles. In the ML models, these profiles are treated as 1D images or features. These ML models identify specific scattering angles, or regions of scattering angles, which correspond with and successfully predict distinct structural parameters. Thus, this work demonstrates that ML strategies can integrate theoretical molecular models with experimental solution scattering data, providing a new framework for extracting highly relevant structural information from solution experiments on biological macromolecules.
Keywords: computational modelling; machine learning; ribonucleic acids; solution X-ray scattering; wide-angle X-ray scattering.
© Chen and Pollack 2020.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources

