4,15-Dimethyl-7,12-diazo-niatri-cyclo-[10.4.0.02,7]hexa-deca-1(12),2,4,6,13,15-hexa-ene dibromide monohydrate
- PMID: 32939301
- PMCID: PMC7472763
- DOI: 10.1107/S2056989020011147
4,15-Dimethyl-7,12-diazo-niatri-cyclo-[10.4.0.02,7]hexa-deca-1(12),2,4,6,13,15-hexa-ene dibromide monohydrate
Abstract
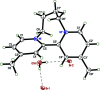
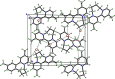
The title compound, C16H20N2 2+·2Br-·H2O (1) is a member of the class of compounds called viologens. Viologens are quaternary salts of di-pyridyls and are especially useful as redox indicators as a result of their large negative one-electron reduction potentials. Compound 1 consists of a dication composed of a pair of 4-methyl-pyridine rings mutually joined at the 2-position, with a dihedral angle between the pyridine rings of 62.35 (4)°. In addition, the rings are tethered via the pyridine nitro-gen atoms by a tetra-methyl-ene bridge. Charge balance is provided by a pair of bromide anions, which are hydrogen bonded to a single water mol-ecule [D O⋯Br = 3.3670 (15) and 3.3856 (15) Å]. The crystal structure of 1, details of an improved synthesis, and a full analysis of its NMR spectra are presented.
Keywords: atropisomer; crystal structure; synthesis; viologen.
© Behrman et al. 2020.
Figures
References
-
- Abraham, M. H. & Grellier, P. L. (1976). J. Chem. Soc. Perkin II 1735–1741.
-
- Alkorta, I., Elguero, J., Roussel, C., Vanthunyne, N. & Piras, P. (2012). Adv. Heterocycl. Chem., 105, 1–188.
-
- Anderson, R. F. & Patel, K. B. (1984). J. Chem. Soc. Faraday Trans. 1, 80, 2693–2702.
-
- Bondi, A. (1964). J. Phys. Chem. 68, 441–451.
-
- Bruker (2016). APEX3 Bruker AXS Inc., Madison, Wisconsin, USA.
LinkOut - more resources
Full Text Sources
Miscellaneous
