Human innate immune cell crosstalk induces melanoma cell senescence
- PMID: 32939325
- PMCID: PMC7470184
- DOI: 10.1080/2162402X.2020.1808424
Human innate immune cell crosstalk induces melanoma cell senescence
Abstract
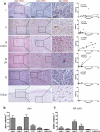
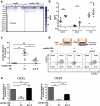
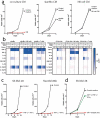
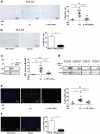
Mononuclear phagocytes and NK cells constitute the first line of innate immune defense. How these cells interact and join forces against cancer is incompletely understood. Here, we observed an early accumulation of slan+ (6-sulfo LacNAc) non-classical monocytes (slanMo) in stage I melanoma, which was followed by an increase in NK cell numbers in stage III. Accordingly, culture supernatants of slanMo induced migration of primary human NK cells in vitro via the chemotactic cytokine IL-8 (CXCL8), suggesting a role for slanMo in NK cell recruitment into cancer tissues. High levels of TNF-α and IFN-γ were produced in co-cultures of TLR-ligand stimulated slanMo and NK cells, whereas much lower levels were contained in cultures of slanMo and NK cells alone. Moreover, TNF-α and IFN-γ concentrations in slanMo/NK cell co-cultures exceeded those in CD14+ monocyte/NK cell and slanMo/T cell co-cultures. Importantly, TNF-α and IFN-γ that was produced in TLR-ligand stimulated slanMo/NK cell co-cultures induced senescence in different melanoma cell lines, as indicated by reduced melanoma cell proliferation, increased senescence-associated β-galactosidase expression, p21 upregulation, and induction of a senescence-associated secretory phenotype (SASP). Taken together, we identified a role for slanMo and NK cells in a collaborative innate immune defense against melanoma by generating a tumor senescence-inducing microenvironment. We conclude that enhancing the synergistic innate immune crosstalk of slanMo and NK cells could improve current immunotherapeutic approaches in melanoma.
Keywords: NK cell; cytokines; melanoma; senescence; slanMo.
© 2020 The Author(s). Published with license by Taylor & Francis Group, LLC.
Figures

References
-
- Schakel K, Mayer E, Federle C, Schmitz M, Riethmuller G, Rieber EP.. A novel dendritic cell population in human blood: one-step immunomagnetic isolation by a specific mAb (M-DC8) and in vitro priming of cytotoxic T lymphocytes. Eur J Immunol. 1998;28:4084–12. doi:10.1002/(SICI)1521-4141(199812)28:12<4084::AID-IMMU4084>3.0.CO;2-4. - DOI - PubMed
-
- Schakel K, von Kietzell M, Hansel A, Ebling A, Schulze L, Haase M, Semmler C, Sarfati M, Barclay AN, Randolph GJ. Human 6-sulfo LacNAc-expressing dendritic cells are principal producers of early interleukin-12 and are controlled by erythrocytes. Immunity. 2006;24:767–777. doi:10.1016/j.immuni.2006.03.020. - DOI - PubMed
-
- Schmitz M, Zhao S, Deuse Y, Schakel K, Wehner R, Wohner H, Hölig K, Wienforth F, Kiessling A, Bornhäuser M, et al. Tumoricidal potential of native blood dendritic cells: direct tumor cell killing and activation of NK cell-mediated cytotoxicity. J Immunol. 2005;174(7):4127–4134. doi:10.4049/jimmunol.174.7.4127. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
