High-Dose Neonatal Vitamin A Supplementation to Bangladeshi Infants Increases the Percentage of CCR9-Positive Treg Cells in Infants with Lower Birthweight in Early Infancy, and Decreases Plasma sCD14 Concentration and the Prevalence of Vitamin A Deficiency at Two Years of Age
- PMID: 32939553
- PMCID: PMC7675026
- DOI: 10.1093/jn/nxaa260
High-Dose Neonatal Vitamin A Supplementation to Bangladeshi Infants Increases the Percentage of CCR9-Positive Treg Cells in Infants with Lower Birthweight in Early Infancy, and Decreases Plasma sCD14 Concentration and the Prevalence of Vitamin A Deficiency at Two Years of Age
Abstract
Background: Vitamin A (VA) stores are low in early infancy and may impair development of the immune system.
Objective: This study determined if neonatal VA supplementation (VAS) affects the following: 1) development of regulatory T (Treg) cells; 2) chemokine receptor 9 (CCR9) expression, which directs mucosal targeting of immune cells; and 3) systemic endotoxin exposure as indicated by changed plasma concentrations of soluble CD14 (sCD14). Secondarily, VA status, growth, and systemic inflammation were investigated.
Methods: In total, 306 Bangladeshi infants were randomly assigned to receive 50,000 IU VA or placebo (PL) within 48 h of birth, and immune function was assessed at 6 wk, 15 wk, and 2 y. Primary outcomes included the following: 1) peripheral blood Treg cells; 2) percentage of Treg, T, and B cells expressing CCR9; and 3) plasma sCD14. Secondary outcomes included the following: 4) VA status measured using the modified relative dose-response (MRDR) test and plasma retinol; 5) infant growth; and 6) plasma C-reactive protein (CRP). Statistical analysis identified group differences and interactions with sex and birthweight.
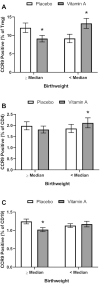
Results: VAS increased (P = 0.004) the percentage of CCR9+ Treg cells (13.2 ± 1.37%) relative to PL (9.17 ± 1.15%) in children below the median birthweight but had the opposite effect (P = 0.04) in those with higher birthweight (VA, 9.13 ± 0.89; PL, 12.1 ± 1.31%) at 6 and 15 wk (values are combined mean ± SE). VAS decreased (P = 0.003) plasma sCD14 (1.56 ± 0.025 mg/L) relative to PL (1.67 ± 0.032 mg/L) and decreased (P = 0.034) the prevalence of VA deficiency (2.3%) relative to PL (9.2%) at 2 y.
Conclusions: Neonatal VAS enhanced mucosal targeting of Treg cells in low-birthweight infants. The decreased systemic exposure to endotoxin and improved VA status at 2 y may have been due to VA-mediated improvements in gut development resulting in improved barrier function and nutrient absorption. This trial was registered at clinicaltrials.gov as NCT01583972 and NCT02027610.
Keywords: B cell; Bangladesh; CCR9; CRP; MRDR test; T cell; Treg cell; neonate; sCD14; vitamin A.
Published by Oxford University Press on behalf of the American Society for Nutrition 2020.
Figures






References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

