Patient perspectives on the therapeutic profile of botulinum neurotoxin type A in cervical dystonia
- PMID: 32939574
- PMCID: PMC7914227
- DOI: 10.1007/s00415-020-10217-7
Patient perspectives on the therapeutic profile of botulinum neurotoxin type A in cervical dystonia
Erratum in
-
Correction to: Patient perspectives on the therapeutic profile of botulinum neurotoxin type A in cervical dystonia.J Neurol. 2021 Mar;268(3):913. doi: 10.1007/s00415-020-10255-1. J Neurol. 2021. PMID: 33104874 Free PMC article. No abstract available.
Abstract
Background: Botulinum neurotoxin type A (BoNT-A) is an effective pharmacological treatment for the management of cervical dystonia (CD) that requires repeated administration at variable intervals. We explored patient perceptions of the impact of CD and the waning of BoNT-A therapeutic effects.
Methods: An internet-based survey was conducted through Carenity, a global online patient community, from May to September 2019. Eligible respondents were adults with CD who had ≥ 2 previous BoNT-A injections.
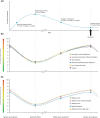
Results: 209 respondents (81% females; mean age of 49.7 years) met the screening criteria. The mean BoNT-A injection frequency was 3.9 injections/year. The mean reported onset of BoNT-A therapeutic effect was 11.7 days and the time to peak effect was 4.5 weeks. Symptom re-emergence between injections was common (88%); the time from injection to symptom re-emergence was 73.6 days (~ 10.5 weeks). Treatment was not reported to completely abolish symptoms, even at peak effect. However, symptom severity was rated (0 = no symptoms; 10 = very strong symptoms) as lowest at the peak of treatment effects (mean scores ~ 3/10), increasing as the effects of treatment start waning (~ 5.5/10) and was strongest one day before the next session (~ 7-8/10). The impact of CD on quality of life followed the same 'rollercoaster' pattern.
Conclusions: This survey highlights the burden of CD symptoms, even in patients undergoing regular treatment. Symptom re-emergence is common and has significant impact on daily activities and quality of life. Greater awareness of the therapeutic profile of BoNT-A treatment should lead to better informed therapeutic discussions and planning.
Keywords: Botulinum toxin; Cervical dystonia; Patient; Survey; Treatment; Waning of effect.
Conflict of interest statement
Dr. Comella serves on the editorial board of Clinical Neuropharmacology and Sleep Medicine. She receives compensation/honoraria for services as a consultant or an advisory committee member: Acorda Therapeutics, Allergan, Inc; Lundbeck Ltd.; Merz Pharmaceuticals; Acadia Pharmaceuticals; Ipsen Pharmaceuticals, Jazz. Pharmaceuticals, Neurocrine Biosciences Inc., Revance Therapeutic, Sunovion., AEON Biopharma. She receives royalties from Cambridge University Press and Wolters Kluwer. She receives research support from the Parkinson’s Disease Foundation. Joaquim J. Ferreira has held consultancy functions with GlaxoSmithKline, Novartis, TEVA, Lundbeck, Solvay, Abbott, Abbvie, BIAL, Merck-Serono, Merz, Ipsen, Biogen, NeuroDerm, Zambon, Sunovion, Affiris, ONO; has received lecture fees from Biogen and BIAL, Sunovion, ONO, Zambon, Abbvie; has received grants from GlaxoSmithKline, Grunenthal, MSD, Allergan, Novartis, Fundação MSD (Portugal), Medtronic and Teva; has been employed by Faculdade de Medicina de Lisboa and CNS—Campus Neurológico Sénior. Marion Azoulai and Emilie Pain are employed by Carenity who received funding from Ipsen for conducting this survey. Savary Om was an Ipsen employee at the time of study.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

