Sex-specific effects of high-fat diet on cognitive impairment in a mouse model of VCID
- PMID: 32939871
- PMCID: PMC7737404
- DOI: 10.1096/fj.202000085R
Sex-specific effects of high-fat diet on cognitive impairment in a mouse model of VCID
Abstract
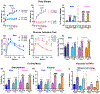
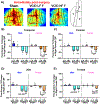
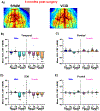
Mid-life metabolic disease (ie, obesity, diabetes, and prediabetes) causes vascular dysfunction and is a risk factor for vascular contributions to cognitive impairment and dementia (VCID), particularly in women. Using middle-aged mice, we modeled metabolic disease (obesity/prediabetes) via chronic high-fat (HF) diet and modeled VCID via unilateral common carotid artery occlusion. VCID impaired spatial memory in both sexes, but episodic-like memory in females only. HF diet caused greater weight gain and glucose intolerance in middle-aged females than males. HF diet alone impaired episodic-like memory in both sexes, but spatial memory in females only. Finally, the combination of HF diet and VCID elicited cognitive impairments in all tests, in both sexes. Sex-specific correlations were found between metabolic outcomes and memory. Notably, both visceral fat and the pro-inflammatory cytokine tumor necrosis factor alpha correlated with spatial memory deficits in middle-aged females, but not males. Overall, our data show that HF diet causes greater metabolic impairment and a wider array of cognitive deficits in middle-aged females than males. The combination of HF diet with VCID elicits deficits across multiple cognitive domains in both sexes. Our data are in line with clinical data, which shows that mid-life metabolic disease increases VCID risk, particularly in females.
Keywords: cerebral blood flow; diet-induced obesity; prediabetes; sex; vascular contributions to cognitive impairment and dementia.
© 2020 Federation of American Societies for Experimental Biology.
Conflict of interest statement
DISCLOSURE/CONFLICT OF INTEREST
The authors have no conflicts to disclose.
Figures
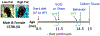



References
-
- Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S, American Heart Association Stroke Council, C. o. E., Prevention, C. o. C. N. C. o. C. R., Intervention, Council on Cardiovascular, S., and Anesthesia. (2011) Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke 42, 2672–2713 - PMC - PubMed
-
- Eringa EC, Serne EH, Meijer RI, Schalkwijk CG, Houben AJ, Stehouwer CD, Smulders YM, and van Hinsbergh VW (2013) Endothelial dysfunction in (pre)diabetes: characteristics, causative mechanisms and pathogenic role in type 2 diabetes. Reviews in endocrine & metabolic disorders 14, 39–48 - PubMed
-
- McCrimmon RJ, Ryan CM, and Frier BM (2012) Diabetes and cognitive dysfunction. Lancet 379, 2291–2299 - PubMed
-
- Biessels GJ, Staekenborg S, Brunner E, Brayne C, and Scheltens P (2006) Risk of dementia in diabetes mellitus: a systematic review. The Lancet. Neurology 5, 64–74 - PubMed
-
- Gannon OJ, Robison LS, Custozzo AJ, and Zuloaga KL (2018) Sex differences in risk factors for vascular contributions to cognitive impairment & dementia. Neurochemistry international - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

