Microglial phagocytosis dysfunction in the dentate gyrus is related to local neuronal activity in a genetic model of epilepsy
- PMID: 32940364
- PMCID: PMC7756777
- DOI: 10.1111/epi.16692
Microglial phagocytosis dysfunction in the dentate gyrus is related to local neuronal activity in a genetic model of epilepsy
Abstract
Objective: Microglial phagocytosis of apoptotic cells is an essential component of the brain regenerative response during neurodegeneration. Whereas it is very efficient in physiological conditions, it is impaired in mouse and human mesial temporal lobe epilepsy, and now we extend our studies to a model of progressive myoclonus epilepsy type 1 in mice lacking cystatin B (CSTB).
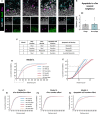
Methods: We used confocal imaging and stereology-based quantification of apoptosis and phagocytosis of the hippocampus of Cstb knockout (KO) mice, an in vitro model of phagocytosis and siRNAs to acutely reduce Cstb expression, and a virtual three-dimensional (3D) model to analyze the physical relationship between apoptosis, phagocytosis, and active hippocampal neurons.
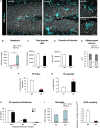
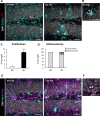
Results: Microglial phagocytosis was impaired in the hippocampus of Cstb KO mice at 1 month of age, when seizures arise and hippocampal atrophy begins. This impairment was not related to the lack of Cstb in microglia alone, as shown by in vitro experiments with microglial Cstb depletion. The phagocytosis impairment was also unrelated to seizures, as it was also present in Cstb KO mice at postnatal day 14, before seizures begin. Importantly, phagocytosis impairment was restricted to the granule cell layer and spared the subgranular zone, where there are no active neurons. Furthermore, apoptotic cells (both phagocytosed and not phagocytosed) in Cstb-deficient mice were at close proximity to active cFos+ neurons, and a virtual 3D model demonstrated that the physical relationship between apoptotic cells and cFos+ neurons was specific for Cstb KO mice.
Significance: These results suggest a complex crosstalk between apoptosis, phagocytosis, and neuronal activity, hinting that local neuronal activity could be related to phagocytosis dysfunction in Cstb KO mice. Overall, these data suggest that phagocytosis impairment is an early feature of hippocampal damage in epilepsy and opens novel therapeutic approaches for epileptic patients based on targeting microglial phagocytosis.
Keywords: apoptosis; epilepsy; hippocampus; microglia; phagocytosis; seizures.
© 2020 The Authors. Epilepsia published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
None of the authors has any conflict of interest to disclose.
Figures



Similar articles
-
Early microglial activation precedes neuronal loss in the brain of the Cstb-/- mouse model of progressive myoclonus epilepsy, EPM1.J Neuropathol Exp Neurol. 2012 Jan;71(1):40-53. doi: 10.1097/NEN.0b013e31823e68e1. J Neuropathol Exp Neurol. 2012. PMID: 22157618
-
Abnormal microglial activation in the Cstb(-/-) mouse, a model for progressive myoclonus epilepsy, EPM1.Glia. 2015 Mar;63(3):400-11. doi: 10.1002/glia.22760. Epub 2014 Oct 18. Glia. 2015. PMID: 25327891
-
Gene-Expression Profiling Suggests Impaired Signaling via the Interferon Pathway in Cstb-/- Microglia.PLoS One. 2016 Jun 29;11(6):e0158195. doi: 10.1371/journal.pone.0158195. eCollection 2016. PLoS One. 2016. PMID: 27355630 Free PMC article.
-
Unverricht-Lundborg disease.Epileptic Disord. 2016 Sep 1;18(S2):28-37. doi: 10.1684/epd.2016.0841. Epileptic Disord. 2016. PMID: 27582036 Review.
-
Progressive Myoclonus Epilepsy: Unverricht-Lundborg Disease.In: Noebels JL, Avoli M, Rogawski MA, Vezzani A, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 5th edition. New York: Oxford University Press; 2024. Chapter 51. In: Noebels JL, Avoli M, Rogawski MA, Vezzani A, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 5th edition. New York: Oxford University Press; 2024. Chapter 51. PMID: 39637163 Free Books & Documents. Review.
Cited by
-
The Roles of Cystatin B in the Brain and Pathophysiological Mechanisms of Progressive Myoclonic Epilepsy Type 1.Cells. 2024 Jan 16;13(2):170. doi: 10.3390/cells13020170. Cells. 2024. PMID: 38247861 Free PMC article. Review.
-
Opioids, microglia, and temporal lobe epilepsy.Front Neurol. 2024 Jan 5;14:1298489. doi: 10.3389/fneur.2023.1298489. eCollection 2023. Front Neurol. 2024. PMID: 38249734 Free PMC article. Review.
-
Microglia Fighting for Neurological and Mental Health: On the Central Nervous System Frontline of COVID-19 Pandemic.Front Cell Neurosci. 2021 Feb 18;15:647378. doi: 10.3389/fncel.2021.647378. eCollection 2021. Front Cell Neurosci. 2021. PMID: 33737867 Free PMC article. Review.
-
A bottom-up approach identifies the antipsychotic and antineoplastic trifluoperazine and the ribose derivative deoxytubercidin as novel microglial phagocytosis inhibitors.Glia. 2025 Feb;73(2):330-351. doi: 10.1002/glia.24637. Epub 2024 Nov 4. Glia. 2025. PMID: 39495090 Free PMC article.
-
Upregulation of adenosine A2A receptor in astrocytes is sufficient to trigger hippocampal multicellular dysfunctions and memory deficits.Mol Psychiatry. 2025 Jul 23. doi: 10.1038/s41380-025-03115-9. Online ahead of print. Mol Psychiatry. 2025. PMID: 40702259
References
-
- Joensuu T, Lehesjoki AE, Kopra O. Molecular background of EPM1‐Unverricht‐Lundborg disease. Epilepsia. 2008;49:557–63. - PubMed
-
- Lalioti MD, Scott HS, Buresi C, Rossier C, Bottani A, Morris MA, et al. Dodecamer repeat expansion in cystatin B gene in progressive myoclonus epilepsy. Nature. 1997;386:847–51. - PubMed
-
- Pennacchio LA, Lehesjoki A‐E, Stone NE, Willour VL, Virtaneva K, Miao J, et al. Mutations in the gene encoding cystatin B in progressive myoclonus epilepsy (EPM1). Science. 1996;271:1731–4. - PubMed
-
- Riccio M, Di Giaimo R, Pianetti S, Palmieri PP, Melli M, Santi S. Nuclear localization of cystatin B, the cathepsin inhibitor implicated in myoclonus epilepsy (EPM1). Exp Cell Res. 2001;262:84–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- PI_2016_1_0011/Eusko Jarlaritza, Department of Education/International
- Ikerbasque Foundation/International
- Folkhälsan Research Foundation/International
- P-048-FTPGB 2018/Fundación Tatiana Pérez de Guzmán el Bueno/International
- IN16,_BBM_BAS_0260/Fundación BBVA/International
- BFU2012-32089/Ministerio de Ciencia e Innovación/International
- BFU2015-66689/Ministerio de Ciencia e Innovación/International
- RTI2018-099267-B-I00/Ministerio de Ciencia e Innovación/International
- RYC-2013-12817/Ministerio de Ciencia e Innovación/International
- SAF2015-69484-R/Ministerio de Ciencia e Innovación/International
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

