A Unified Strategy for Arylsulfur(VI) Fluorides from Aryl Halides: Access to Ar-SOF3 Compounds
- PMID: 32940381
- PMCID: PMC7756513
- DOI: 10.1002/anie.202009699
A Unified Strategy for Arylsulfur(VI) Fluorides from Aryl Halides: Access to Ar-SOF3 Compounds
Abstract
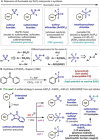
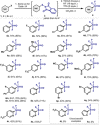
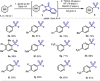
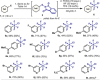
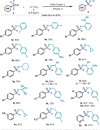
A convenient protocol to selectively access various arylsulfur(VI) fluorides from commercially available aryl halides in a divergent fashion is presented. Firstly, a novel sulfenylation reaction with the electrophilic N-(chlorothio)phthalimide (Cl-S-Phth) and arylzinc reagents afforded the corresponding Ar-S-Phth compounds. Subsequently, the S(II) atom was selectively oxidized to distinct fluorinated sulfur(VI) compounds under mild conditions. Slight modifications on the oxidation protocol permit the chemoselective installation of 1, 3, or 4 fluorine atoms at the S(VI) center, affording the corresponding Ar-SO2 F, Ar-SOF3 , and Ar-SF4 Cl. Of notice, this strategy enables the effective introduction of the rare and underexplored -SOF3 moiety into various (hetero)aryl groups. Reactivity studies demonstrate that such elusive Ar-SOF3 can be utilized as a linchpin for the synthesis of highly coveted aryl sulfonimidoyl fluorides (Ar-SO(NR)F).
Keywords: heterocycles; sulfinyl trifluorides; sulfonimidoyl fluorides; sulfonyl fluorides; tetrafluorosulfanyl chlorides.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- None
-
- Lu X., Yi Q., Pan X., Wang P., Vessally E. J., J. Sulfur Chem. 2020, 41, 210–228;
-
- Wu R., Huang K., Qiu G., Liu J.-B., Synthesis 2019, 51, 3567–3587;
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

