The 2-Pyridyl Problem: Challenging Nucleophiles in Cross-Coupling Arylations
- PMID: 32940402
- PMCID: PMC8246887
- DOI: 10.1002/anie.202010631
The 2-Pyridyl Problem: Challenging Nucleophiles in Cross-Coupling Arylations
Abstract
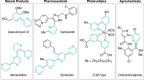
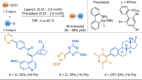
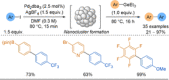
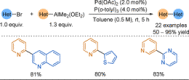
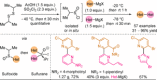
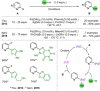
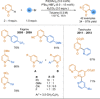
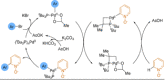
Azine-containing biaryls are ubiquitous scaffolds in many areas of chemistry, and efficient methods for their synthesis are continually desired. Pyridine rings are prominent amongst these motifs. Transition-metal-catalysed cross-coupling reactions have been widely used for their synthesis and functionalisation as they often provide a swift and tuneable route to related biaryl scaffolds. However, 2-pyridine organometallics are capricious coupling partners and 2-pyridyl boron reagents in particular are notorious for their instability and poor reactivity in Suzuki-Miyaura cross-coupling reactions. The synthesis of pyridine-containing biaryls is therefore limited, and methods for the formation of unsymmetrical 2,2'-bis-pyridines are scarce. This Review focuses on the methods developed for the challenging coupling of 2-pyridine nucleophiles with (hetero)aryl electrophiles, and ranges from traditional cross-coupling processes to alternative nucleophilic reagents and novel main group approaches.
Keywords: biaryl; catalysis; palladium; pyridine; synthetic methods.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
































Similar articles
-
A highly efficient precatalytic system (XPhos-PdG2) for the Suzuki-Miyaura cross-coupling of 7-chloro-1H-pyrrolo[2,3-c]pyridine employing low catalyst loading.Mol Divers. 2019 Aug;23(3):697-707. doi: 10.1007/s11030-018-9904-6. Epub 2019 Jan 9. Mol Divers. 2019. PMID: 30627855
-
A Bulky Chiral N-Heterocyclic Carbene Palladium Catalyst Enables Highly Enantioselective Suzuki-Miyaura Cross-Coupling Reactions for the Synthesis of Biaryl Atropisomers.J Am Chem Soc. 2019 Sep 18;141(37):14938-14945. doi: 10.1021/jacs.9b08578. Epub 2019 Sep 6. J Am Chem Soc. 2019. PMID: 31460761
-
Palladium-catalysed cross-coupling reaction of ultra-stabilised 2-aryl-1,3-dihydro-1H-benzo[d]1,3,2-diazaborole compounds with aryl bromides: A direct protocol for the preparation of unsymmetrical biaryls.Beilstein J Org Chem. 2014 May 13;10:1107-13. doi: 10.3762/bjoc.10.109. eCollection 2014. Beilstein J Org Chem. 2014. PMID: 24991260 Free PMC article.
-
Applications of and alternatives to pi-electron-deficient azine organometallics in metal catalyzed cross-coupling reactions.Chem Soc Rev. 2007 Jul;36(7):1058-68. doi: 10.1039/b616082d. Epub 2007 Mar 2. Chem Soc Rev. 2007. PMID: 17576474 Review.
-
Synthesis of Symmetrical and Nonsymmetrical Polyenes by Iterative and Bidirectional Palladium-Catalyzed Cross-Coupling Reactions.Chemistry. 2020 Oct 27;26(60):13543-13567. doi: 10.1002/chem.202000624. Epub 2020 Sep 17. Chemistry. 2020. PMID: 32267574 Review.
Cited by
-
Halide Salts Alleviate TMSOK Inhibition in Suzuki-Miyaura Cross-Couplings.ACS Catal. 2024 Aug 7;14(16):12671-12680. doi: 10.1021/acscatal.4c02407. eCollection 2024 Aug 16. ACS Catal. 2024. PMID: 39169912 Free PMC article.
-
Unconventional Site Selectivity in Palladium-Catalyzed Cross-Couplings of Dichloroheteroarenes under Ligand-Controlled and Ligand-Free Systems.J Org Chem. 2022 Jun 3;87(11):7414-7421. doi: 10.1021/acs.joc.2c00665. Epub 2022 May 18. J Org Chem. 2022. PMID: 35584051 Free PMC article.
-
Photochemically Mediated Ring Expansion of Indoles and Pyrroles with Chlorodiazirines: Synthetic Methodology and Thermal Hazard Assessment.Angew Chem Int Ed Engl. 2023 Aug 1;62(31):e202305081. doi: 10.1002/anie.202305081. Epub 2023 Jun 27. Angew Chem Int Ed Engl. 2023. PMID: 37294032 Free PMC article.
-
Functionalized in Triplicate: A Ring-By-Ring Approach to Tailored Prodiginine Derivatives for Site-Specific Conjugation Through Click Chemistry.Chemistry. 2025 Aug 1;31(43):e202502066. doi: 10.1002/chem.202502066. Epub 2025 Jul 14. Chemistry. 2025. PMID: 40626904 Free PMC article.
-
Cycloparaazine, a full-azine carbon nanoring.Nat Commun. 2025 Jun 9;16(1):4643. doi: 10.1038/s41467-025-59934-5. Nat Commun. 2025. PMID: 40490467 Free PMC article.
References
-
- None
-
- Funk A., Divekar P. V., Can. J. Microbiol. 1959, 5, 317–321; - PubMed
-
- Kiuru P., Yli-Kauhaluoma J., Adv. Heterocycl. Nat. Prod. Synth. 2011, 267–297.
-
- None
-
- Bekiari V., Lianos P., Judeinstein P., Chem. Phys. Lett. 1999, 307, 310–316;
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

