The 2-Pyridyl Problem: Challenging Nucleophiles in Cross-Coupling Arylations
- PMID: 32940402
- PMCID: PMC8246887
- DOI: 10.1002/anie.202010631
The 2-Pyridyl Problem: Challenging Nucleophiles in Cross-Coupling Arylations
Abstract
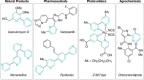
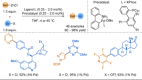
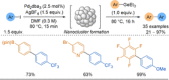
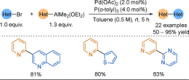
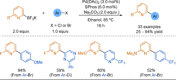
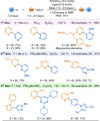
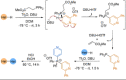
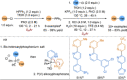
Azine-containing biaryls are ubiquitous scaffolds in many areas of chemistry, and efficient methods for their synthesis are continually desired. Pyridine rings are prominent amongst these motifs. Transition-metal-catalysed cross-coupling reactions have been widely used for their synthesis and functionalisation as they often provide a swift and tuneable route to related biaryl scaffolds. However, 2-pyridine organometallics are capricious coupling partners and 2-pyridyl boron reagents in particular are notorious for their instability and poor reactivity in Suzuki-Miyaura cross-coupling reactions. The synthesis of pyridine-containing biaryls is therefore limited, and methods for the formation of unsymmetrical 2,2'-bis-pyridines are scarce. This Review focuses on the methods developed for the challenging coupling of 2-pyridine nucleophiles with (hetero)aryl electrophiles, and ranges from traditional cross-coupling processes to alternative nucleophilic reagents and novel main group approaches.
Keywords: biaryl; catalysis; palladium; pyridine; synthetic methods.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









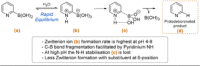

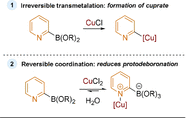

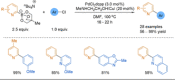




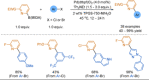










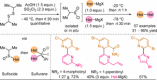

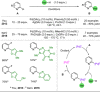




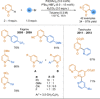
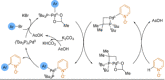

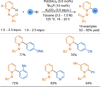

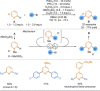
References
-
- None
-
- Funk A., Divekar P. V., Can. J. Microbiol. 1959, 5, 317–321; - PubMed
-
- Kiuru P., Yli-Kauhaluoma J., Adv. Heterocycl. Nat. Prod. Synth. 2011, 267–297.
-
- None
-
- Bekiari V., Lianos P., Judeinstein P., Chem. Phys. Lett. 1999, 307, 310–316;
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

