Multicenter evaluation of two chemiluminescence and three lateral flow immunoassays for the diagnosis of COVID-19 and assessment of antibody dynamic responses to SARS-CoV-2 in Taiwan
- PMID: 32940547
- PMCID: PMC7580576
- DOI: 10.1080/22221751.2020.1825016
Multicenter evaluation of two chemiluminescence and three lateral flow immunoassays for the diagnosis of COVID-19 and assessment of antibody dynamic responses to SARS-CoV-2 in Taiwan
Abstract
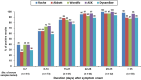
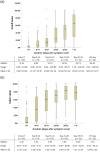
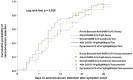
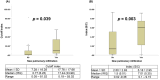
This multicenter, retrospective study included 346 serum samples from 74 patients with coronavirus disease 2019 (COVID-19) and 194 serum samples from non-COVID-19 patients to evaluate the performance of five anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody tests, i.e. two chemiluminescence immunoassays (CLIAs): Roche Elecsys® Anti-SARS-CoV-2 Test (Roche Test) and Abbott SARS-CoV-2 IgG (Abbott Test), and three lateral flow immunoassays (LFIAs): Wondfo SARS-CoV-2 Antibody Test (Wondfo Test), ASK COVID-19 IgG/IgM Rapid Test (ASK Test), and Dynamiker 2019-nCoV IgG/IgM Rapid Test (Dynamiker Test). We found high diagnostic sensitivities (%, 95% confidence interval [CI]) for the Roche Test (97.4%, 93.4-99.0%), Abbott Test (94.0%, 89.1-96.8%), Wondfo Test (91.4%, 85.8-94.9%), ASK Test (97.4%, 93.4-99.0%), and Dynamiker Test (90.1%, 84.3-94.0%) after >21 days of symptom onset. Meanwhile, the diagnostic specificity was 99.0% (95% CI, 96.3-99.7%) for the Roche Test, 97.9% (95% CI, 94.8-99.2%) for the Abbott Test, and 100.0% (95% CI, 98.1-100.0%) for the three LFIAs. Cross-reactivity was observed in sera containing anti-cytomegalovirus (CMV) IgG/IgM antibodies and autoantibodies. No difference was observed in the time to seroconversion detection of the five serological tests. Specimens from patients with COVID-19 pneumonia demonstrated a shorter seroconversion time and higher chemiluminescent signal than those without pneumonia. Our data suggested that understanding the dynamic antibody response after COVID-19 infection and performance characteristics of different serological test are crucial for the appropriate interpretation of serological test result for the diagnosis and risk assessment of patient with COVID-19 infection.
Keywords: COVID-19; antibody response; chemiluminescence immunoassays; cross-reactivity; lateral flow immunoassays.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

References
-
- WHO Statement (31 January 2020). “Statement on the second meeting of the International Health Regulations . (2005). Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV). World Health Organization. Retrieved 6 February 2020. Available from: https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-....
-
- World Health Organization . Coronavirus disease (COVID-2019) situation reports – 166 [cited 15 Jul 2020]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situatio....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
