Traube-Hering waves are formed by interaction of respiratory sinus arrhythmia and pulse pressure modulation in healthy men
- PMID: 32940558
- PMCID: PMC7790131
- DOI: 10.1152/japplphysiol.00452.2020
Traube-Hering waves are formed by interaction of respiratory sinus arrhythmia and pulse pressure modulation in healthy men
Abstract
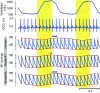
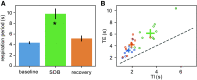
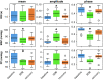
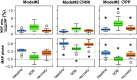
Excessive blood pressure variation is linked to the development of hypertension and other diseases. This study assesses the relative role of respiratory sinus arrhythmia (RSA) and pulse pressure (PP) on the amplitude and timing of blood pressure variability with respiration [Traube-Hering (TH) waves]. We analyzed respiratory, electrocardiogram, and blood pressure traces from healthy, supine male subjects (n = 10, mean age = 26.7 ± 1.4) during 20-min epochs of resting, slow deep breathing (SDB), and recovery. Across all epochs, blood pressure and heart rate (HR) were modulated with respiration and the magnitude of RSA; TH waves increased during SDB. The data were deconstructed using a simple mathematical model of blood pressure to dissect the relative roles of RSA and PP on TH waves. We constructed the time series of the R-wave peaks and compared the recorded TH waves with that predicted by the model. Given that cardiac output is determined by both heart rate and stroke volume, it was surprising that the magnitude of the TH waves could be captured by only HR modulation. However, RSA alone did not accurately predict the timing of TH waves relative to the respiratory cycle. Adding respiratory modulation of PP to the model corrected the phase shift showing the expected pattern of BP rising during inspiration with the peak of the TH wave during early expiration. We conclude that short-term variability of blood pressure referred to as TH waves has at least two independent mechanisms whose interaction forms their pattern: RSA and respiratory-driven changes in PP.NEW & NOTEWORTHY Variability in blood pressure has become an important metric to consider as more is learned about the link between excessive blood pressure variability and adverse health outcomes. In this study using slow deep breathing in human subjects, we found that heart rate and pulse pressure variations have comparable effects on the amplitude of blood pressure waves, and it is the common action of the two that defines the phase relationship between respiration and blood pressure oscillations.
Keywords: blood pressure; cardiorespiratory coupling; heart rate variability; pulse pressure.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


References
-
- Chenniappan M. Blood pressure variability: assessment, prognostic significance and management. J Assoc Physicians India 63: 47–53, 2015. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

