Alkylative damage of mRNA leads to ribosome stalling and rescue by trans translation in bacteria
- PMID: 32940602
- PMCID: PMC7521929
- DOI: 10.7554/eLife.61984
Alkylative damage of mRNA leads to ribosome stalling and rescue by trans translation in bacteria
Abstract
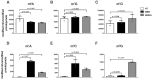
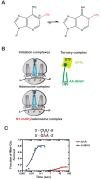
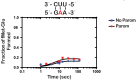
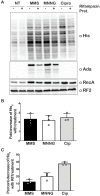
Similar to DNA replication, translation of the genetic code by the ribosome is hypothesized to be exceptionally sensitive to small chemical changes to its template mRNA. Here we show that the addition of common alkylating agents to growing cultures of Escherichia coli leads to the accumulation of several adducts within RNA, including N(1)-methyladenosine (m1A). As expected, the introduction of m1A to model mRNAs was found to reduce the rate of peptide bond formation by three orders of magnitude in a well-defined in vitro system. These observations suggest that alkylative stress is likely to stall translation in vivo and necessitates the activation of ribosome-rescue pathways. Indeed, the addition of alkylation agents was found to robustly activate the transfer-messenger RNA system, even when transcription was inhibited. Our findings suggest that bacteria carefully monitor the chemical integrity of their mRNA and they evolved rescue pathways to cope with its effect on translation.
Keywords: E. coli; RNA damage; alkylation; biochemistry; chemical biology; quality control; ribosome; tmRNA; trans translation.
© 2020, Thomas et al.
Conflict of interest statement
ET, KK, EM, TM, HZ No competing interests declared
Figures



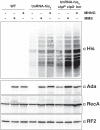
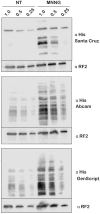
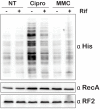
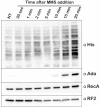
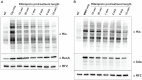


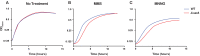
References
-
- Brandman O, Stewart-Ornstein J, Wong D, Larson A, Williams CC, Li GW, Zhou S, King D, Shen PS, Weibezahn J, Dunn JG, Rouskin S, Inada T, Frost A, Weissman JS. A ribosome-bound quality control complex triggers degradation of nascent peptides and signals translation stress. Cell. 2012;151:1042–1054. doi: 10.1016/j.cell.2012.10.044. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

