Changes in Anthropometric Parameters After Anti-TNFα Therapy in Inflammatory Bowel Disease: A Systematic Review and Meta-analysis
- PMID: 32940873
- PMCID: PMC7519901
- DOI: 10.1007/s40259-020-00444-9
Changes in Anthropometric Parameters After Anti-TNFα Therapy in Inflammatory Bowel Disease: A Systematic Review and Meta-analysis
Abstract
Background: Tumour necrosis factor (TNF)-α inhibitors have been widely used for the treatment of moderate-to-severe inflammatory bowel disease (IBD). TNFα also plays an important role in the regulation of weight homeostasis and metabolism and has been linked to variations in anthropometric responses. This relationship in patients with IBD has yet to be determined.
Objectives: Our objective was to evaluate the effects of TNFα inhibitors on changes in anthropometric measures in both adults and children with IBD through a systematic review and meta-analysis.
Methods: Multiple database searches identified studies involving children and adults with IBD and treated with TNFα inhibitors and reporting at least one primary outcome measure. Where possible, data were combined for meta-analysis. The primary outcomes included weight, body mass index (BMI), waist circumference, height, height/velocity, and fat and lean mass. Secondary outcomes included surrogate markers of disease activity. A random-effects model was used to estimate the standardised mean difference (SMD).
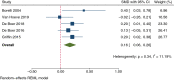
Results: In total, 23 cohort studies (total 1167 participants) met the inclusion criteria. Meta-analysis was performed on 13 of these studies. In children, 6-29.3 months of anti-TNFα therapy had a small but statistically significant effect on weight (SMD 0.31; 95% confidence interval [CI] 0.12-0.49; P = 0.001) with a mean gain in z score of 0.30 (standard error [SE] 0.12). In adults, 2-22.4 months of treatment had a moderate effect on BMI (SMD 0.72; 95% CI 0.17-1.26; P = 0.010; mean gain 1.23 kg/m2; SE 0.21). A small but statistically significant increase in BMI z score was found in children (SMD 0.28; 95% CI 0.03-0.53; P = 0.026; mean change 0.31 ± standard deviation [SD] 0.14) after 12-29.3 months of therapy. A meta-analysis of four studies found a negligible but statistically significant increase in height (SMD 0.16; 95% CI 0.06-0.26; P = 0.002; mean change 0.17 z score [SE 0.05]). A negligible effect on fat mass (SMD 0.24; 95% CI -0.19-0.66; P = 0.272) was found in a meta-analysis of five studies. Of note, despite the high heterogeneity among the studies that addressed the issue, these results were also consistently supported by findings from studies not included in the meta-analysis and reviewed in the systematic review. Unfortunately, a lack of data meant we were unable to perform moderator analysis on observed heterogeneity.
Conclusion: Anti-TNFα treatment appears to be associated with an increase in body weight, BMI, and other anthropometric parameters. Given the differing courses of IBD between children and adults, this association should be considered before initiating biologics for undernourished, overweight, and obese patients. Registration: PROSPERO registration number CRD42020163079.
Conflict of interest statement
Faizan Mazhar, Vera Battini, Marco Pozzi, Elena Invernizzi, Giulia Mosini, Michele Gringeri, Annalisa Capuano, Cristina Scavone, Sonia Radice, Emilio Clementi, and Carla Carnovale have no conflicts of interest that are directly relevant to the content of this article.
Figures




References
-
- Sfriso P, Caso F, Filardo GS, Botsios C, Costa L, Scarpa R, et al. Impact of 24 months of anti-TNF therapy versus methotrexate on body weight in patients with rheumatoid arthritis: a prospective observational study. Clin Rheumatol. 2016;35(6):1615–1618. doi: 10.1007/s10067-016-3244-7. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

