Fortified Coiled Coils: Enhancing Mechanical Stability with Lactam or Metal Staples
- PMID: 32940968
- PMCID: PMC7821110
- DOI: 10.1002/anie.202006971
Fortified Coiled Coils: Enhancing Mechanical Stability with Lactam or Metal Staples
Abstract
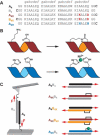
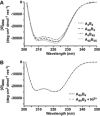
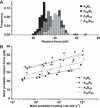
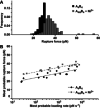
Coiled coils (CCs) are powerful supramolecular building blocks for biomimetic materials, increasingly used for their mechanical properties. Here, we introduce helix-inducing macrocyclic constraints, so-called staples, to tune thermodynamic and mechanical stability of CCs. We show that thermodynamic stabilization of CCs against helix uncoiling primarily depends on the number of staples, whereas staple positioning controls CC mechanical stability. Inserting a covalent lactam staple at one key force application point significantly increases the barrier to force-induced CC dissociation and reduces structural deformity. A reversible His-Ni2+ -His metal staple also increases CC stability, but ruptures upon mechanical loading to allow helix uncoiling. Staple type, position and number are key design parameters in using helical macrocyclic templates for fine-tuning CC properties in emerging biomaterials.
Keywords: coiled coil; lactam; metal coordination; peptide stapling; single-molecule force spectroscopy.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

