Sulfono-γ-AApeptides as Helical Mimetics: Crystal Structures and Applications
- PMID: 32940995
- PMCID: PMC8903043
- DOI: 10.1021/acs.accounts.0c00482
Sulfono-γ-AApeptides as Helical Mimetics: Crystal Structures and Applications
Abstract
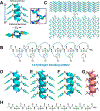
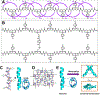
Foldamers have defined and predictable structures, improved resistance to proteolytic degradation, enhanced chemical diversity, and are versatile in their mimicry of biological molecules, making them promising candidates in biomedical and material applications. However, as natural macromolecules exhibit endless folding structures and functions, the exploration of the applications of foldamers remains crucial. As such, it is imperative to continue to discover unnatural foldameric architectures with new frameworks and molecular scaffolds. To this end, we recently developed a new class of peptidomimetics termed ″γ-AApeptides", oligomers of γ-substituted-N-acylated-N-aminoethyl amino acids, which are inspired by the chiral peptide nucleic acid backbone. To date γ-AApeptides have been shown to be resistant to proteolytic degradation and possess limitless potential to introduce chemically diverse functional groups, demonstrating promise in biomedical and material sciences. However, the structures of γ-AApeptides were initially unknown, rendering their rational design for the mimicry of a protein helical domain impossible in the beginning, which limited their potential development. To our delight, in the past few years, we have obtained a series of crystal structures of helical sulfono-γ-AApeptides, a subclass of γ-AApeptides. The single-crystal X-ray crystallography indicates that sulfono-γ-AApeptides fold into unprecedented and well-defined helices with unique helical parameters. On the basis of the well-established size, shape, and folding conformation, the design of sulfono-γ-AApeptide-based foldamers opens a new avenue for the development of alternative unnatural peptidomimetics for their potential applications in chemistry, biology, medicine, materials science, and so on.In this Account, we will outline our journey on sulfono-γ-AApeptides and their application as helical mimetics. We will first briefly introduce the design and synthetic strategy of sulfono-γ-AApeptides and then describe the crystal structures of helical sulfono-γ-AApeptides, including left-handed homogeneous sulfono-γ-AApeptides, right-handed 1:1 α/sulfono-γ-AA peptide hybrids, and right-handed 2:1 α/sulfono-γ-AA peptide hybrids. After that, we will illustrate the potential of helical sulfono-γ-AApeptides for biological applications such as the disruption of medicinally relevant protein-protein interactions (PPIs) of BCL9-β-catenin and p53-MDM2/MDMX as well as the mimicry of glucagon-like peptide 1 (GLP-1). In addition, we also exemplify their potential application in material science. We expect that this Account will shed light on the structure-based design and function of helical sulfono-γ-AApeptides, which can provide a new and alternative way to explore and generate novel foldamers with distinctive structural and functional properties.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
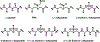
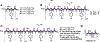


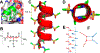


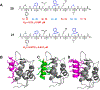






Similar articles
-
Inhibition of β-catenin/B cell lymphoma 9 protein-protein interaction using α-helix-mimicking sulfono-γ-AApeptide inhibitors.Proc Natl Acad Sci U S A. 2019 May 28;116(22):10757-10762. doi: 10.1073/pnas.1819663116. Epub 2019 May 14. Proc Natl Acad Sci U S A. 2019. PMID: 31088961 Free PMC article.
-
Right-Handed Helical Foldamers Consisting of De Novo d-AApeptides.J Am Chem Soc. 2017 May 31;139(21):7363-7369. doi: 10.1021/jacs.7b03007. Epub 2017 May 16. J Am Chem Soc. 2017. PMID: 28480699 Free PMC article.
-
Sulfono-γ-AApeptides as a new class of nonnatural helical foldamer.Chemistry. 2015 Feb 2;21(6):2501-7. doi: 10.1002/chem.201406112. Epub 2014 Dec 11. Chemistry. 2015. PMID: 25504756
-
Helical sulfono-γ-AApeptides with predictable functions in protein recognition.RSC Chem Biol. 2022 May 20;3(7):805-814. doi: 10.1039/d2cb00049k. eCollection 2022 Jul 6. RSC Chem Biol. 2022. PMID: 35866163 Free PMC article. Review.
-
Unnatural helical peptidic foldamers as protein segment mimics.Chem Soc Rev. 2023 Jul 31;52(15):4843-4877. doi: 10.1039/d2cs00395c. Chem Soc Rev. 2023. PMID: 37401344 Free PMC article. Review.
Cited by
-
α/Sulfono-γ-AApeptide Hybrid Analogues of Glucagon with Enhanced Stability and Prolonged In Vivo Activity.J Med Chem. 2021 Sep 23;64(18):13893-13901. doi: 10.1021/acs.jmedchem.1c01289. Epub 2021 Sep 10. J Med Chem. 2021. PMID: 34506138 Free PMC article.
-
α/Sulfono-γ-AA peptide hybrids agonist of GLP-1R with prolonged action both in vitro and in vivo.Acta Pharm Sin B. 2023 Apr;13(4):1648-1659. doi: 10.1016/j.apsb.2022.10.014. Epub 2022 Oct 21. Acta Pharm Sin B. 2023. PMID: 37139407 Free PMC article.
-
Harnessing Aromatic-Histidine Interactions through Synergistic Backbone Extension and Side Chain Modification.Angew Chem Int Ed Engl. 2023 Oct 2;62(40):e202308100. doi: 10.1002/anie.202308100. Epub 2023 Aug 29. Angew Chem Int Ed Engl. 2023. PMID: 37587780 Free PMC article.
-
Geared Toward Applications: A Perspective on Functional Sequence-Controlled Polymers.ACS Macro Lett. 2021 Feb 16;10(2):243-257. doi: 10.1021/acsmacrolett.0c00855. Epub 2021 Jan 20. ACS Macro Lett. 2021. PMID: 34336395 Free PMC article.
-
Development of the Safe and Broad-Spectrum Aldehyde and Ketoamide Mpro inhibitors Derived from the Constrained α, γ-AA Peptide Scaffold.Chemistry. 2023 Jun 22;29(35):e202300476. doi: 10.1002/chem.202300476. Epub 2023 May 5. Chemistry. 2023. PMID: 36920943 Free PMC article.
References
-
- Guichard G; Huc I Synthetic foldamers. Chem. Commun. 2011, 47 (21), 5933–5941. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

